Abstract
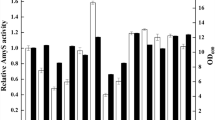
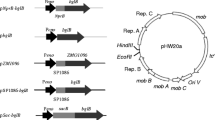
By using a β-glucanase from Bacillus as a model protein, we investigated whether the secretion competence based on the action of the kil gene can be improved using stronger promoters for the expression of the kil gene. Since the production of extracellular target proteins also depends on the promoter strengths of the target gene, we constructed four expression vectors with all possible combinations of a weak and a strong stationary-phase promoter for the kil gene, and a weak and a strong constitutive promoter, respectively, for the β-glucanase gene. The results of batch fermentations showed that the use of stronger promoters generally decreased the cell density. However, a drastic increase of productivity of the cells to produce and secrete β-glucanase resulted in a significantly higher activity of extracellular β-glucanase. The yield of extracellular β-glucanase can be increased (to 168 %) by using a strong promoter for the β-glucanase alone. However, the increase was much higher when the weak promoter of the kil gene was replaced by a strong stationary-phase promoter (to 221 %). An even higher yield of extracellular β-glucanase was reached when β-glucanase was expressed by a strong promoter in addition indicating a combinatorial effect. This shows that the extracellular production of a recombinant target gene can be optimized by tuning the promoter strengths of components, the kil gene and the target gene.





Similar content being viewed by others
References
Becker G, Hengge-Aronis R (2001) What makes an Escherichia coli promoter σs-dependent? Role of the −13/−14 nucleotide promoter positions and region 2.5 of σs. Mol Microbiol 39:1153–1165
Beshay U, Miksch G, Friehs K, Flaschel E (2007a) Improved β-glucanase production by a recombinant Escherichia coli strain using zinc-ion supplemented medium. Eng Life Sci 7:253–258
Beshay U, Miksch G, Flaschel E (2007b) Improvement of a β-glucanase activity test by taking into account the batch reactor balance of the test system. Bioprocess Biosyst Eng (in-press). DOI 10.1007/s00449-007-0121-4
Beshay U, Miksch G, Friehs K, Flaschel E (2003a) Production of a bacterial β-glucanase by expression in Escherichia coli and simultaneous adsorption on a metal chelate affinity resin. Arab J Biotechnol 6:183–190
Beshay U, Friehs K, Azzam A, Flaschel E (2003b) Cultivation of Dictyostelium discodeum in immobilized form by colonization of porous supports. Proc Biochem 38:1521–1529
Blanchin-Roland S, Masson JM (1989) Protein secretion controlled by a synthetic gene in Escherichia coli. Protein Eng 2:473–480
Borris R, Olsen O, Thomsen KKK, von Wettstein D (1989) Hybrid Bacillus endo-(1–3,1–4)-β-glucanases: construction of recombinant genes and molecular properties of the gene product. Carlsberg Res Commun 54:41–54
Cavard D (1991) Synthesis and functioning of the colicin E1 lysis protein: Comparison with the colicin A lysis protein. J Bacteriol 173:191–196
Fellay R, Frey J, Krisch H (1987) Interposon mutagensis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of Gram-negative bacteria. Gene 52:147–154
Figge J, Wright C, Collins CJ, Roberts TM, Livingston DM (1988) Stringent regulation of stably integrated chloramphenicol acetyl transferase genes by E. coli lac repressor in monkey cells. Cell 52:713–722
Jensen PR, Hammer K (1998) The sequence of spacers between the consensus sequences modulates the strength of prokaryotic promoters. Appl Env Microbiol 64:82–87
Koshla C, Bailey JE (1989) Evidence for partial export of Vitreoscilla hemoglobin into the periplasmic space in Escherichia coli. J Mol Biol 210:79–80
Makrides SC (1996) Strategies for achieving high-level expression of genes in Escherichia coli. Microbiol Rev 60:512–538
Miksch G, Flaschel E (2001) Secretion of homologous and heterologous recombinant proteins in Escherichia coli and other gram-negative bacteria by using a new secretion system. In: Merten O-W, Mattanovich D, Larsson G, Cole JA, Lang C, Neubauer P, Porro D, Teixeira de Mattos J (eds) Recombinant protein production with prokaryotic and eukaryotic cells. A comparative view on host physiology. Kluwer, Amsterdam, Netherlands, pp 347–358
Miksch G, Bettenworth F, Friehs K, Flaschel E (2005) The sequence upstream of the—10 consensus sequence modulates the strength and induction time of stationary-phase promoters in Escherichia coli. Appl Microbiol Biotechnol 69:312–320
Miksch G, Fiedler E, Dobrowolski P, Friehs K (1997a) The kil gene of the CoIE1 plasmid of Escherichia coli controlled by a growth-phase-dependent promoter mediates the secretion of a heterologous periplasmic protein during the stationary phase. Arch Microbiol 167:143–150
Miksch G, Fiedler E, Dobrowolski P, Flaschel E (1997b) Controlled secretion into the culture medium of a hybrid β-glucanase by Acetobacter methanolicus mediated by the kil gene of Escherichia coli located on a Tn5-derived transposon. Appl Microbiol Biotechnol 47:530–536
Miksch G, Kleist S, Friehs K, Flaschel E (2002) Overexpression of the phytase from Escherichia coli and its extracellular production in bioreactors. Appl Microbiol Biotechnol 59:685–694
Miksch G, Neitzel R, Fiedler R, Friehs K, Flaschel E (1997c) Extracellular production of a hybrid β-glucanase from Bacillus by Escherichia coli under different cultivation conditions in shaking cultures and bioreactors. Appl Microbiol Biotechnol 47:120–126
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring harbor Laboratory Press, Cold Spring Harbor, N.Y
Acknowledgements
U. Beshay is grateful to the Alexander von Humboldt-Foundation for the financial support in the form of a scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Beshay, U., Miksch, G., Friehs, K. et al. Increasing the secretion ability of the kil gene for recombinant proteins in Escherichia coli by using a strong stationary-phase promoter . Biotechnol Lett 29, 1893–1901 (2007). https://doi.org/10.1007/s10529-007-9477-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-007-9477-4