Abstract
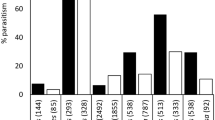
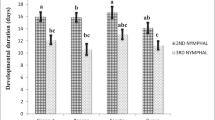
The population dynamics of Cotesia urabae (Austin and Allen) (Braconidae: Microgastrinae), a biological control agent from Tasmania, and its eucalypt feeding host, Uraba lugens (Walker) (Lepidoptera: Nolidae) was investigated prior to its introduction to New Zealand in 2011. Previous host range testing on potential New Zealand non-targets determined C. urabae had some potential to attack an endemic species, Nyctemera annulata (Boisduval) (Lepidoptera: Arctiidae). A closely related species in Tasmania, Nyctemera amica, was thus investigated as a potential host along with the native host U. lugens, to better understand the host range of C. urabae and the synchrony with its host in Tasmania. Adult C. urabae emerged from pupal cocoons in the field during January which confirmed a five month window in which its host, the larvae of U. lugens, was absent in the field. Experiments using sentinel N. amica and U. lugens larvae, field collections of N. amica and of larvae of other Lepidopteran species during this five month time window detected no parasitism by C. urabae. In the laboratory, host specificity testing showed reduced attack rates and no resultant C. urabae eggs or developing larvae or any successful pupation of C. urabae larvae from attacked N. amica larvae. It was concluded that N. amica is most unlikely to be a host for C. urabae in Tasmania and no evidence of any other alternative host was found.

Similar content being viewed by others
References
Akhurst RJ, James W, Bird LJ, Beard C (2003) Resistance to the Cry1Ac delta-endotoxin of Bacillus thuringiensis in the cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). J Econ Entomol 96:1290–1299
Allen GR (1990a) Influence of host behaviour and host size on the success of oviposition of Cotesia urabae and Dolichogenidea eucalypti (Hymenoptera: Braconidae). J Insect Behav 3:733–749
Allen GR (1990b) The phenologies of Cotesia urabae, Dolichogenidea eucalypti (Hymenoptera: Braconidae) and their host Uraba lugens (Lepidoptera: Noctuidae) in the Adelaide region. Aust J Zool 38:347–362
Allen GR (1990c) Uraba lugens Walker (Lepidoptera: Noctuidae): larval survival and parasitoid biology in the field in South Australia. J Aust Entomogical Soc 29:301–312
Allen GR, Keller MA (1991) Uraba lugens (Lepidoptera: Noctuidae) and its parasitoids (Hymenoptera: Braconidae): temperature, host size, and development. Environ Entomol 20:458–469
Austin AD, Allen GR (1989) Parasitoids of Uraba lugens Walker (Lepidoptera: Noctuidae) in South Australia, with description of two new species of Braconidae. Trans R Soc South Aust 113:169–184
Benn M, DeGrave J, Gnanasunderam C, Hutchins R (1979) Host–plant pyrrolizidine alkaloids in Nyctemera annulata Boisduval: their persistence through the life cycle and transfer to a parasite. Experientia 35:731–732
Berndt LA, Allen GR (2010) Biology and pest status of Uraba lugens Walker (Lepidoptera: Nolidae) in Australia and New Zealand. Aust J Entomol 49:268–277
Berndt LA, Mansfield S, Withers TM (2007) A method for host range testing of a biological control agent for Uraba lugens. New Zealand Plant Prot 60:286–290
Berndt LA, Withers T, Mansfield S, Hoare RJB (2009) Non-target species selection for host range testing of Cotesia urabae. New Zealand Plant Prot 62:168–173
Brimblecombe AR (1962) Outbreaks of the Eucalypt leaf skeletonizer. Qld J Agric Sci 19:208–217
Cameron PJ, Walker GP and Keller MA (1998) Comparative measures of the host specificity of Cotesia rubecula and Cotesia plutellae (Hymenoptera: Braconidae). Sixth Australasian Applied Entomological Research Conference, pp. 511–515
Campbell KG (1962) The biology of Roeselia lugens (Walk.), the gum-leaf skeletonizer moth, with particular reference to the Eucalyptus camaldulensis Dehn. (River Red Gum) forests of the Murray River region. Proceedings of the Linnean Society of New South Wales 87, pp. 316–338
Clarke AR (1996) Parasitoids associated with a Tasmanian population of Nyctemera amica (White) (Lepidoptera: Arctiidae). Aust Entomol 23:17–20
Danilevs AS, Goryshin NI, Tyshchen VP (1970) Biological rhythms in terrestrial arthropods. Annu Rev Entomol 15:201–244
Elliott HJ and de Little DW (1984) Insect pests of trees and timber in Tasmania, Hobart: Forestry Commission, Tasmania, p. 90
Endersby NM and Cameron PJ (2004) Parasitism of Nyctemera amica (White) (Lepidoptera: Arctiidae) and Plutella xylostella (L.) (Lepidoptera: Plutellidae) by Cotesia plutellae (Kurdjumov) (Hymenoptera: Braconidae). In: Endersby NM, Ridland PM (eds) The management of diamondback moth and other crucifer pests: Proceedings of the fourth international workshop, Melbourne, Australia, pp. 265–268
Farr JD (2002) Biology of the gum leaf skeletonizer, Uraba lugens Walker (Lepidoptera: Noctuidae), in the southern jarrah forest of Western Australia. Aust J Entomol 41:60–69
Gibb AR, Suckling DM, Fielder S, Bunn B, Jamieson LE, Larsen ML, Walter GH, Kriticos DJ (2008) Major sex pheromone components of the Australian gum leaf skeletonizer Uraba lugens: (10E, 12Z)-Hexadecadien-1-yl Acetate and (10E, 12Z)-Hexadecadien-1-ol. J Chem Ecol 34:1125–1133
Godfray HCJ (1994) Parasitoids: behavioural and evolutionary ecology. Princeton University Press, New Jersey, USA
Harris JA, Neumann FG and Ward B (1977) An outbreak of the gum leaf skeletonizer, Uraba lugens Walker, in river red gum forest near Barmah. Forest Commission, Victoria, Research Branch Report
Jerman EJ, Gauld I (1988) Casinaria, a paraphyletic ichneumonid genus (Hymenoptera), and a revision of the Australian species. J Nat Hist 22:589–609
Kay M (1980) Nyctemera amica × Nyctemera annulata colony at Woodhill (Lepidoptera, Arctiidae). New Zealand Entomol 7:154–158
Kriticos DJ, Potter KJB, Alexander NS, Gibb AR, Suckling M (2007) Using a pheromone lure survey to establish the native and potential distribution of an invasive Lepidopteran, Uraba lugens. J Appl Ecol 44:853–863
Krunic MD, Stanisavljevic LZ (2006) Supercooling points and diapause termination in overwintering adults of orchard bees Osmia cornuta and O-rufa (Hymenoptera: Megachilidae). Bull Entomol Res 96:323–326
Marktl RC (2002) Interspecific competition between the braconid endoparasitoids Glyptapanteles porthetriae and Glyptapanteles liparidis in Lymantria dispar larvae. Entomol Exp Appl 105:97–109
Polgar LA, Hardie J (2000) Diapause induction in aphid parasitoids. Entomol Exp Appl 97:21–27
Strelein GJ (1988) Gum leaf skeletonizer moth, Uraba lugens, in the forests of Western Australia. Aust For 51:197–204
Suckling M, Gibb AR, Dentener PR, Seldon DS, Clare GK, Jamieson L, Kriticos DJ, El-Sayed AM (2005) Uraba lugens (Lepidoptera: Nolidae) in New Zealand: pheromone trapping for delimitation and phenology. J Econ Entomol 98:1187–1192
Tatsumi E, Takada H (2005) Effects of photoperiod and temperature on adult oligopause of Aphelinus asychis and larval diapause of A. albipodus (Hymenoptera: Aphelinidae). Appl Entomol Zool 40:447–456
van Klinken RD, Edwards OR (2002) Is host-specificity of weed biological control agents likely to evolve rapidly following establishment? Ecol Lett 5:590–596
Acknowledgments
This project was financially supported by Scion (New Zealand Forest Research Institute Ltd.) through the School of Agricultural Science, at the University of Tasmania. We would like to thank Forestry Tasmania for supplying seedlings for our laboratory colony and the Co-operative Research Centre for Forestry for providing the necessary space and equipment, without which this would not have been possible. Special thanks go to Vin Patel and Steve Quarrell for their assistance collecting plant and insect materials.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Dirk Babendreier.
Rights and permissions
About this article
Cite this article
Rowbottom, R.M., Allen, G.R., Walker, P.W. et al. Phenology, synchrony and host range of the Tasmanian population of Cotesia urabae introduced into New Zealand for the biocontrol of Uraba lugens . BioControl 58, 625–633 (2013). https://doi.org/10.1007/s10526-013-9524-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-013-9524-0