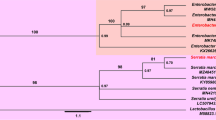
Abstract
Surveys for biological control agents of the invasive weed Schinus terebinthifolius (Anacardiaceae) discovered two Omolabus weevils (Coleoptera: Attelabidae) feeding on the plant in its native range. Molecular and morphological analysis indicated that one of these species consistently fed on the target weed and the other species fed more broadly. Aspects of the biology and host range of the more specific species, Omolabus piceus (Germar) were examined to determine its suitability as a biological control agent of S. terebinthifolius in the USA. Adults feed on newly formed leaves, and eggs, larvae and pupae develop in curled fragments of leaves, called nidi. Larvae consumed an average of 11.3 (±0.4) mg throughout their development which required 15.1 (±0.2) days. An average of 31.6 (±2.7) eggs were laid per female during their 23.8 (±2.2) day lifetime, after a 3.4 (±1.0) day preoviposition period. In no-choice tests, O. piceus adults fed and oviposited on all tested native North American, Caribbean and agricultural Anacardiaceae species except for M. indica. The field host-range of O. piceus, as determined by samples of host use in the native range, included three Schinus, two Lithrea and one Anacardium species. Therefore, we do not recommend O. piceus for biological control of S. terebinthifolius in the USA. However, the utilization of this species in other infested areas such as Hawaií and Australia should be considered.


Similar content being viewed by others
References
Barkley FA (1944) Schinus L. Brittonia 5:160–198
Barkley FA (1957) A study of Schinus L. Lilloa 28:5–110
Bennett FD, Crestana L, Habeck DH, Berti-Filho E (1990) Brazilian peppertree—prospects for biological control. In: Delfosse ES (ed) Proceedings of the VII international symposium on biological control of weeds. Instituto Sperimentale per la Patologia Vegetale, Rome, Italy, pp 293–297
Bertheau C, Schuler H, Krumbock S, Arthofer W, Stauffer C (2011) Hit or miss in phylogeographic analyses: the case of the cryptic NUMTs. Mol Ecol Res 11:1056–1059
Berthier K, Chapuis MP, Moosavi SM, Tohidi-Esfahani D, Sword GA (2011) Nuclear insertions and heteroplasmy of mitochondrial DNA as two sources of intra-individual genomic variation in grasshoppers. Syst Entomol 36:285–299
Calvignac S, Konecny L, Malard F, Douady CJ (2011) Preventing the pollution of mitochondrial datasets with nuclear mitochondrial paralogs (numts). Mitochondrion 11:246–254
Costa Lima AM (1956) Insetos do Brasil. Tomo 10, Coleópteros, 4a e última parte. Escola Nacional de Agronomia. Universidade Federal Rural do Rio de Janeiro, Brazil, pp 1–373
Davis DR, Mc Kay F, Oleiro M, Vitorino MD, Wheeler GS (2011) Biology and systematics of the leafmining Gracillariidae of Brazilian pepper tree, Schinus terebinthifolius Raddi, with descriptions of a new genus and four new species. J Lepidopterists Soc 65:61–93
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797
Ewel J (1986) Invasibility: lessons from South Florida. In: Mooney HA, Drake JA (eds) Ecology of biological invasions of North America and Hawaii. Springer-Verlag, New York, USA, pp 214–230
FLEPPC Plant List Committee (2009) Florida exotic pest plant council’s 2009 list of invasive species. Wild Weeds 12:13–16
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Gaskin JF, Bon MC, Cock MJW, Cristofaro M, Biase AD, De Clerck-Floate R, Ellison CA, Hinz HL, Hufbauer RA, Julien MH, Sforza R (2011) Applying molecular-based approaches to classical biological control of weeds. Biol Control 58:1–21
Goolsby JA, De Barro PJ, Makinson J, Pemberton RW, Hartley DM, Frohlich DR (2006) Matching the origin of an invasive weed for selection of a herbivore haplotype for a biological control programme. Mol Ecol 15:287–297
Hamilton RW (2005) Omolabus Jekel in north and central America (Coleoptera: Attelabidae). Zootaxa 986:1–60
Hight SD, Cuda JP, Medal JC (2002) Brazilian peppertree. In: Lyon S, Blossey B, Hoddle MS, Reardon R, van Driesche RG (eds) Biological control of invasive plants in the eastern United States. USDA Forest Service, Morgantown, USA, pp 311–321
HSASC (2001) Hawaii State Alien Species Coordinator. Department of Land & Natural Resources and Division of Forestry & Wildlife Honolulu. http://www.state.hi.us/dlnr/dofaw/hortweeds/specieslist.htm. Accessed 10 Dec 2012
Iwata K (1935) On the habits of some Rhynchitinae, Attelabinae and Apoderine in Japan (in Japanese). Kontyu 9:261–278
JBRJ (2011) Instituto de Pesquisas Jardim Botanico do Rio de Janeiro. Jabot - Bando de Dados da Flora Brasileira. http://www.jbrj.gov.br/jabot. Accessed 10 Dec 2012
Jessup LW(2012) Anacardiaceae. Flora of Australia online; Australian Biological Resources Study, Canberra. http://www.environment.gov.au/biodiversity/abrs/online-resources/flora/main/index.html. Accessed 10 Dec 2012
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Kobayashi C, Kato M (2004) To be suspended or to be cut off? Differences in the performance of two types of leaf-rolls constructed by the attelabid beetle Cycnotrachelus roelofsi. Pop Ecol 46:193–202
Kobayashi C, Fukasawa Y, Hirose D, Kato M (2007) Contribution of symbiotic mycangial fungi to larval nutrition of a leaf-rolling weevil. Evol Ecol 22:711–722
Li X, Wheeler GS, Ding J (2012) A leaf-rolling weevil benefits from general saprophytic fungi in polysaccharide degradation. Arthropod Plant Interact 6:417–424
Mc Kay F, Oleiro M, Walsh GC, Gandolfo D, Wheeler GS (2009) Natural enemies of Brazilian peppertree (Schinus terebinthifolius: Anacardiaceae) from Argentina: their possible use for biological control in the USA. Florida Entomol 92:292–303
Morton JF (1978) Brazilian pepper—its impact on people, animals and the environment. Econ Bot 32:353–359
Mound LA, Wheeler GS, Williams DA (2010) Resolving cryptic species with morphology and DNA; thrips as a potential biocontrol agent of Brazilian peppertree, with a new species and overview of Pseudophilothrips (Thysanoptera). Zootaxa 2432:59–68
Muñoz JD (2000) Anacardiaceae. In: Hunziker AT (ed) Flora fanerogámica Argentina. Conicet Cordoba, Argentina, pp 1–28
NYBG (2011) New York Botanical Garden. http://www.nybg.org. Accessed 10 Dec 2012
O’Brien CW, Wibmer GJ (1982) Annotated checklist of the weevils (Curculionidae sensu lato) of North America, Central America, and the West Indies (Coleoptera: Curculionidae). The American Entomological Institute, Ann Arbor
Palmer WA (1999) The use of cut foliage instead of whole plants for host specificity testing of weed biocontrol insects—is it acceptable practice? In: Withers TM, Barton-Browne L, Stanley JN (eds) Host specificity testing in Australasia: towards improved assays for biological control. CRC for Tropical Pest Management, Brisbane, Australia, pp 20–23
Panetta FD, McKee J (1997) Recruitment of the invasive ornamental, Schinus terebinthifolius, is dependent upon frugivores. Aust J Ecol 22:432–438
Pell SK, Mitchell JD, Miller AJ, Lobova TA (2011) Anacardiaceae. In: Kubitzki K (ed) The families and genera of vascular plants. Springer-Verlag, New York, USA, pp 7–50
Randall JM (2000) Schinus terebinthifolius Raddi. In: Randall JM, Hoshovsky MC, Bossard CC (eds) Invasive plants of California’s wildlands. University of California Press, Berkeley, USA, pp 282–287
Rector BG, De Biase A, Cristofaro M, Primerano S, Belvedere S, Antonini G, Sobhian R (2010) DNA fingerprinting to improve data collection efficiency and yield in an open-field host-specificity test of a weed biological control candidate. Invasive Plant Sci Manag 3:429–439
SAS Institute (1990) SAS/STAT user’s guide. SAS Institute, Cary, USA
Smith MA, Rodriguez JJ, Whitfield JB, Deans AR, Janzen DH, Hallwachs W, Hebert PDN (2008) Extreme diversity of tropical parasitoid wasps exposed by iterative integration of natural history, DNA barcoding, morphology, and collections. PNAS 105:12359–12364
Song H, Buhay JE, Whiting MF, Crandall KA (2008) Many species in one: DNA barcoding overestimates the number of species when nuclear mitochondrial pseudogenes are coamplified. PNAS 105:13486–13491
Sunnucks P, Hales DF (1996) Numerous transposed sequences of mitochondrial cytochrome oxidase I–II in aphids of the genus Sitobion (Hemiptera: Aphididae). Mol Biol Evol 13:510–524
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tropicos.org (2011) Missouri Botanical Garden. http://www.tropicos.org. Accessed 10 Dec 2012
USDA/NRCS (2002) The PLANTS database. National Plant Data Team, Greensboro, NC 27401-4901, USA. http://plants.usda.gov. Accessed 10 Dec 2012
Wagner WL, Herbst DR, Sohmer SH (1999) Manual of the flowering plants of Hawaii. Bernice pauahi bishop museum special publication, vol 2. Hawaii Press, Honolulu, USA, pp 1–1948
Wang Y, Ding J, Wheeler GS, Purcell MF, Zhang G (2009) Heterapoderopsis bicallosicollis (Coleoptera: Attelabidae): a potential biological control agent for Triadica sebifera. Environ Entomol 38:1135–1144
Wang Y, Wu K, Ding J (2010) Host specificity of Euops chinesis, a potential biological control agent of Fallopia japonica, an invasive plant in Europe and North America. BioControl 55:551–559
Yoshioka ER, Markin GP (1991) Efforts of biological control of Christmas berry Schinus terebinthifolius in Hawaii. In: Center TD, Doren RF, Hofstetter RL, Myers RL, Whiteaker LD (eds) Dept interior. National Park Service, Miami, USA, pp 377–385
Zar JH (1996) Biostatistical analysis. Prentice Hall, New Jersey, USA
Acknowledgments
We wish to thank K. Dyer, USDA/ARS/IPRL for laboratory assistance and Dr. Hans-Joachim Esser (Botanische Staatssammlung München) who assisted in G. klotzschiana identification. Weevil identifications were provided by Dr. C. O’Brien, Taxonomic consultant, Green Valley, AZ, USA. This manuscript was improved by the helpful comments J. Briano, Fundación para el Estudio de Especies Invasivas and two anonymous reviewers. Brazilian insect collections were conducted under the Instituto Brasileiro do Meio Ambiente permits 07BR001027/DF, 08BR002120/DF, 09BR003939/DF, and 10BR004731/DF issued to MV. Insects were introduced under quarantine with a USDA/APHIS permit P526P-07-06609 issued to GSW. Voucher specimens are deposited in the Florida State Collection of Arthropods, DPI, FDACS, Gainesville, FL, USA; and USDA Systematic Entomology Laboratory, Beltsville, MD, USA. This project was partially funded by Florida Fish and Wildlife Conservation Commission, South Florida Water Management District, and United State Department of Agriculture, Agricultural Research Service.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: John Scott.
Rights and permissions
About this article
Cite this article
Wheeler, G.S., Mc Kay, F., Vitorino, M.D. et al. Biology and host range of Omolabus piceus, a weevil rejected for biological control for Schinus terebinthifolius in the USA. BioControl 58, 693–702 (2013). https://doi.org/10.1007/s10526-013-9523-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-013-9523-1