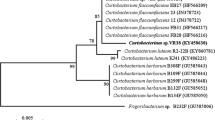
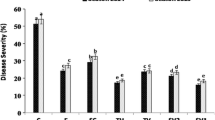
Abstract
Grapevine is one of the most important economic crops yielding berries, wine products as well as derivates. However, due to the large array of pathogens inducing diseases on this plant, considerable amounts of pesticides—with possible negative impact on the environment and health—have been used and are currently used in viticulture. To avoid negative impacts of such products and to ensure product quality, a substantial fraction of pesticides needs to be replaced in the near future. One solution can be related to the use of beneficial bacteria inhabiting the rhizo- and/or the endosphere of plants. These biocontrol bacteria and their secondary metabolites can reduce directly or indirectly pathogen diseases by affecting pathogen performance by antibiosis, competition for niches and nutrients, interference with pathogen signaling or by stimulation of host plant defenses. Due to the large demand for biocontrol of grapevine diseases, such biopesticides, their modes of actions and putative consequences of their uses need to be described. Moreover, the current knowledge on new strains from the rhizo- and endosphere and their metabolites that can be used on grapevine plants to counteract pathogen attack needs to be discussed. This is in particular with regard to the control of root rot, grey mould, trunk diseases, powdery and downy mildews, pierce’s disease, grapevine yellows as well as crown gall. Future prospects on specific beneficial microbes and their secondary metabolites that can be used as elicitors of plant defenses and/or as biocontrol agents with potential use in a more sustainable viticulture will be further discussed.

Similar content being viewed by others
References
Abdalla MA, Win HY, Islam MT, von Tiedemann A, Schuffler A, Laatsch H (2011) Khatmiamycin, a motility inhibitor and zoosporicide against the grapevine downy mildew pathogen Plasmopara viticola from Streptomyces sp. ANK313. J Antibiot 64:655–659
Agüero CB, Uratsu SL, Greve C, Powell ALT, Labavitch JM, Meredith CP, Dandekar AM (2005) Evaluation of tolerance to Pierce’s disease and Botrytis in transgenic plants of Vitis vinifera L. expressing the pear PGIP gene. Mol Plant Pathol 6:43–51
Ait Barka E, Belarbi A, Hachet C, Nowak J, Audran JC (2000) Enhancement of in vitro growth and resistance to gray mould of Vitis vinifera co-cultured with plant growth promoting rhizobacteria. FEMS Microbiol Lett 186:91–95
Ait Barka E, Gognies S, Nowak J, Audran JC, Belarbi A (2002) Inhibitory effect of bacteria on Botrytis cinerea and its influence to promote the grapevine growth. Biol Control 24:135–142
Alfonzo A, Conigliaro G, Torta L, Burruano S, Moschetti G (2009) Antagonism of Bacillus subtilis strain AG1 against vine wood fungal pathogens. Phytopathol Med 48:155–158
Allègre M, Héloir M-C, Trouvelot S, Daire X, Pugin A, Wendehenne D, Adrian M (2009) Are grapevine stomata involved in the elicitor-induced protection against downy mildew? Mol Plant-Microbe Interact 22:977–986
Almeida RPP, Wistrom C, Hill BL, Hashim J, Purcell AH (2005) Vector transmission of Xylella fastidiosa to dormant grape. Plant Dis 89:419–424
Amaro P, Mexia A (2003) The pesticides very toxic to man, to natural enemies, to honey bees and to aquatic life must be prohibited or rigorously restricted for IPM in viticulture. Bull OILB/SROP 26:277–282
Auger J, Esterio M, Ricke G, Pérez I (2004) Black dead arm and basal cankers of Vitis vinifera cv. Red Globe caused by Botryosphaeria obtusa in Chile. Plant Dis 88:1286
Baumgartner K, Warren JG (2005) Persistence of Xylella fastidiosa in riparian hosts near northern California vineyards. Plant Dis 89:1097–1102
Bell CR, Dickie GA, Harvey WLG, Chan JWYF (1995) Endophytic bacteria in grapevine. Can J Microbiol 41:46–53
Bent E (2006) Induced systemic resistance mediated by plant growth-promoting rhizobacteria (PGPR) and fungi (PGPF). In: Tuzun S, Bent E (eds) Multigenic and induced systemic resistance in plants. Springer, New York, USA, pp 225–258
Berg G (2009) Plant-microbe interactions promoting plant growth and health: perspectives for controlled use of microorganisms in agriculture. Appl Microbiol Biotechnol 84:11–18
Bester W, Crous PW, Fourie PH (2007) Evaluation of fungicides as potential grapevine pruning wound protectants against Botryosphaeria species. Aust J Plant Pathol 36:73–77
Bulgari D, Casati P, Crepaldi P, Daffonchio D, Quaglino F, Brusetti L, Bianco PA (2001) Restructuring of endophytic bacterial communities in grapevine yellows-diseased and recovered Vitis vinifera L. plants. Appl Environ Microbiol 77:5018–5022
Burr TJ, Otten L (1999) Crown gall of grape: biology and disease management. Annu Rev Phytopathol 37:53–80
Burr TJ, Reid CL (1994) Biological control of grape crown gall with nontumorigenic Agrobacterium vitis strain F2/5. Am J Enol Vitic 45:213–219
Burr TJ, Reid CL, Tagliati E, Bazzi C, Süle S (1997) Biological control of grape crown gall by strain F2/5 is not associated with agrocin production or competition for attachment sites on grape cells. Phytopathology 87:706–711
Carter MV (1991) The status of Eutypa lata as a pathogen. monograph—phytopathological paper no. 32. International Mycological Institute, Surrey, UK
Carter MV, Price TV (1974) Biological control of Eutypa armeniacae. II. Studies of the interaction between E. armeniacae and Fusarium lateritium, and their relative sensitivities to benzimidazole chemicals. Aust J Agric Res 25:105–119
Castillo-Pando M, Somers A, Green CD, Priest M, Sriskanthades M (2001) Fungi associated with dieback of Semillon grapevines in the Hunter Valley of New South Wales. Aust Plant Pathol 30:59–63
Chatterjee S, Almeida RPP, Lindow SE (2008) Living in two worlds: the plant and insect lifestyles of Xylella fastidiosa. Annu Rev Phytopathol 46:243–271
Chen F, Guo YB, Wang JH, Li JY, Wang HM (2007) Biological control of grape crown gall by Rahnella aquatilis HX2. Plant Dis 91:957–963
Chen F, Li JY, Guo YB, Wang JH, Wang HM (2009) Biological control of grapevine crown gall: purification and partial characterisation of an antibacterial substance produced by Rahnella aquatilis strain HX2. Eur J Plant Pathol 124:427–437
Chiarappa L (2000) Esca (black measles) of grapevine. An overview. Phytopathol Med 39:11–15
Christen D, Tharin M, Perrin-Cherioux S, Abou-Mansour E, Tabacchi R, Defago G (2005) Transformation of eutypa dieback and esca disease pathogen toxins by antagonistic fungal strains reveals a second detoxification pathway not present in Vitis vinifera. J Agric Food Chem 53:7043–7051
Compant S (2011) Editorial : lutte biologique des agents pathogènes de la vigne avec des bactéries symbiotiques, une avancée pour nos vignobles ? Rev Oenol 140:7–8
Compant S, Mathieu F (2011) Biocontrol des maladies de la vigne avec des microbes bénéfiques. Une alternative à la chimie de synthèse en viticulture. Rev Oenol 141(hors série):25
Compant S, Duffy B, Nowak J, Clément C, Ait Barka E (2005a) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71:4951–4959
Compant S, Reiter B, Sessitsch A, Nowak J, Clément C, Ait Barka E (2005b) Endophytic colonization of Vitis vinifera L. by plant growth-promoting bacterium Burkholderia sp. strain PsJN. Appl Environ Microbiol 71:1685–1693
Compant S, Kaplan H, Sessitsch A, Nowak J, Ait Barka E, Clément C (2008a) Endophytic colonization of Vitis vinifera L. by Burkholderia phytofirmans strain PsJN: from the rhizosphere to inflorescence tissues. FEMS Microbiol Ecol 63:84–93
Compant S, Nowak J, Coenye T, Clément C, Ait Barka E (2008b) Diversity and occurrence of Burkholderia spp. in the natural environment. FEMS Microbiol Rev 32:607–626
Compant S, Clément C, Sessitsch A (2010a) Plant growth-promoting bacteria in the rhizo- and endosphere of plants. Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol Biochem 42:669–678
Compant S, van der Heijden M, Sessitsch A (2010b) Climate change effects on beneficial plant-microbes interactions. FEMS Microbiol Ecol 73:197–214
Compant S, Mathieu F, Lebrihi A (2011) Biocontrol de Botrytis cinerea par des bactéries bénéfiques de la rhizosphère ou de l’endosphère. Rev Oenol 141:15–16
Compant S, Sessitsch A, Mathieu F (2012) The 125th anniversary for the first postulation of soil derived endophytic bacteria—a tribute to MLV Galippe. Plant Soil 356:299–301
Constable FE (2010) Phytoplasma epidemiology: grapevines as a model. In: Weintraub PG, Jones P (eds) Phytoplasmas: genomes, plant hosts and vectors. CAB International, Oxfordshire, UK, pp 188–212
Creasap J, Reid C, Goffinet M, Aloni R, Ullrich C, Burr T (2005) Effect of wound position, auxin, and Agrobacterium vitis strain F2/5 on wound healing and crown gall in grapevine. Phytopathology 95:362–367
D`Amelio R R, Berta G, Gamalero E, Massa N, Avidano L, Cantamessa S, D`Agostino G, Bosco D, Marzachi C (2011) Increased plant tolerance against chrysanthemum yellows phytoplasma (Candidatus Phytoplasma asteris) following double inoculation with Glomus mosseae BEG12 and Pseudomonas putida S1Pf1Rif. Plant Pathol 60:1014–1022
Andrade ER de (1993) Ocorrência de espécies do gênero Fusarium em solo cultivado com videira (Vitis spp.) em Santa Catarina. Fitopatol Brasil 18:502–505 (Abstract in Rev Plant Pathol 74:657)
Eastwell KC, Sholberg PL, Sayler RJ (2006) Characterizing potential bacterial biocontrol agents for suppression of Rhizobium vitis, causal agent of crown gall disease in grapevines. Crop Prot 25:1191–1200
Escobar MA, Dandekar AM (2003) Agrobacterium tumefaciens as an agent of disease. Trends Plant Sci 8:380–386
FAOSTAT (2011) http://faostat.fao.org
Ferreira JHS, Matthee FN, Thomas AC (1991) Biological control of Eutypa lata on grapevine by an antagonistic strain of Bacillus subtilis. Phytopathology 81:283–287
Ferreira RB, Monteiro SS, Piçarra-Pereira MA, Teixeira AR (2004) Engineering grapevine for increased resistance to fungal pathogens without compromising wine stability. Trends Biotechnol 22:168–173
Ficke A, Gadoury DM, Seem RC (2002) Ontogenic resistance and plant disease management: a case study of grape powdery mildew. Phytopathology 92:671–675
Fourie P, Halleen F (2006) Chemical and biological protection of grapevine propagation material. Eur J Plant Pathol 116:255–265
Gadoury DM, Cadle-Davidson L, Wilcox WF, Dry IB, Seem RC, Milgroom MG (2012) Grapevine powdery mildew (Erysiphe necator): a fascinating system for the study of the biology, ecology and epidemiology of an obligate biotroph. Mol Plant Pathol 13:1–16
Gamalero E, D’Amelio R, Musso C, Cantamessa S, Pivato B, D’Agostino G, Duan J, Bosco D, Marzachi C, Berta G (2010) Effects of Pseudomonas putida S1Pf1Rif against Chrysanthemum yellows Phytoplasma infection. Phytopathology 100:805–813
Gessler C, Pertot I, Perazzolli M (2011) Plasmopara viticola: a review of knowledge on downy mildew of grapevine and effective disease management. Phytopathol Med 50:3–44
Gouadec D, Blouin J (2007) Les parasites de la vigne. Stratégies de protection raisonnée. Dunod, France
Gramaje D, Armengol J (2011) Fungal trunk pathogens in the grapevine propagation process: potential inoculum sources, detection, identification and management strategies. Plant Dis 95:1040–1055
Granett J, Omer AD, Pessereau P, Walker MA (1998) Fungal infections of grapevine roots in phylloxera-infested vineyards. Vitis 37:39–42
Graniti A, Surico G, Mugnai L (2000) Esca of grapevine: a disease complex or a complex of diseases? Phytopathol Mediterr 39:16–20
Grasso S (1984) Infezioni di Fusarium oxysporum e di Cylindrocarpon destructans associate a una moria di giovani piante di vite in Sicilia. Inform Fitopatol 34:59–63
Gubler WD, Baumgartner K, Browne GT, Eskalen A, Latham SR, Petit E, Bayramian LA (2004) Root diseases of grapevines in California and their control. Aust Plant Pathol 33:157–165
Gubler WD, Rolshausen PE, Trouillas FP, Úrbez-Torres JR, Voegel T, Leavitt GM, Weber EA (2005) Grapevine trunk diseases in California. Pract Winery Vineyard (Jan/Feb):6–25
Gugino BK, Travis JW, Stewart EL (2001) Pathogenicity of Fusarium spp., Cylindrocarpon sp. and a Diplodina sp. on grape roots. Phytopathology 91(Suppl)S34
Guo YB, Li J, Li L, Chen F, Wu W, Wang J, Wang H (2009) Mutations that disrupt either the pqq or the gdh gene of Rahnella aquatilis abolish the production of an antibacterial substance and result in reduced biological control of grapevine crown gall. Appl Environ Microbiol 75:6792–6803
Halleen F, Fourie PH, Lombard PJ (2010) Protection of grapevine pruning wounds against Eutypa lata by biological and chemical methods. S Afr J Enol Vitic 31:125–132
Hallmann J (2001) Plant interactions with endophytic bacteria. In: Jeger MJ, Spence NJ (eds) Biotic interactions in plant–pathogen associations. CABI Publishing, Wallingford, UK, pp 87–119
Hallmann J, Berg B (2007) Spectrum and population dynamics of bacterial root endophytes. In: Schulz BJE, Boyle CJC, Sieber TN (eds) Microbial root endophytes. Springer, Berlin, Germany, pp 15–31
Hartmann A, Rothballer M, Schmid M (2008) Lorenz Hiltner, a pioneer in rhizosphere microbial ecology and soil bacteriology research. Plant Soil 312:7–14
Highet AS, Nair NG (1995) Fusarium oxysporum associated with grapevine decline in the Hunter Valley, NSW, Australia. Aust J Grape Wine Res 1:48–50
Hiltner L (1904) Über neue Erfahrungen und Probleme auf dem Gebiet der Bodenbakteriologie und unter besonderer Berücksichtigung der Gründüngung und Brache. Arb Deutsch Landwirtschafts-Gesellschaft 98:59–78
Hopkins DL (1989) Xylella fastidiosa: xylem-limited bacterial pathogen of plants. Annu Rev Phytopathol 27:271–290
Hopkins DL (2005) Biological control of Pierce’s disease in the vineyard with strains of Xylella fastidiosa benign to grapevine. Plant Dis 89:1348–1352
John S, Scott ES, Wicks TJ, Hunt JS (2004) Interaction between Eutypa lata and Trichoderma harzianum. Phytopathol Med 43:95–104
Kawaguchi A (2008) Biological control of crown gall of grapevine, rose, and tomato by nonpathogenic Agrobacterium vitis strain VAR03-1. Phytopathology 98:1218–1225
Kawaguchi A, Inoue K, Nasu H (2005) Inhibition of crown gall formation by Agrobacterium radiobacter biovar 3 strains isolated from grapevine. J Gen Plant Pathol 71:422–430
Kawaguchi A, Inoue K, Nasu H (2007) Biological control of grapevine crown gall by nonpathogenic Agrobacterium vitis strain VAR03-1. J Gen Plant Pathol 73:133–138
Khmel IA, Sorokina TA, Lemanova NB, Lipasova VA, Metlitski OZ, Burdeinaya TV, Chernin LS (1998) Biological control of crown gall in grapevine and raspberry by two Pseudomonas spp. with a wide spectrum of antagonistic activity. Bio Sci Technol 8:45–57
Kiss L (2003) A review of fungal antagonists of powdery mildews and their potential as biocontrol agents. Pest Man Sci 59:475–483
Kloepper JW, Ryu C-M, Zhang S (2004) Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 94:1259–1266
Kotze C (2008) Biological control of the grapevine trunk disease pathogens: pruning wound protection. Master thesis, University of Stellenbosch, South Africa
Krol E (1998) Epiphytic bacteria isolated from grape leaves and its effect on Botrytis cinerea Pers. Phytopathol Pol 16:53–61
Larignon P, Dubos B (2001) The villainy of black dead arm. Wines Vines 82:86–89
LdR Garrido, Sônego OR, Gomes VN (2004) Fungi associated with grapevine showing decline and plant death in the state of Rio Grande do Sul, Southern Brazil. Fitopatol Bras 29:322–324
Leavitt GM (1990) The occurrence, distribution, effects and control of Botryodiplodia theobromae on Vitis vinifera in California, Arizona and northern Mexico. Ph.D. dissertation, University of California, Riverside, USA
Lebrihi A, Errakhi R, Barakate M (2009a) New Streptomyces barakatei strain, culture filtrate, derived active compounds and use thereof in the treatment of plants. Patent WO/2009/156687
Lebrihi A, Errakhi R, Barakate M (2009b) New Streptomyces beta-vulgaris strain, culture filtrate, derived active compounds and use thereof in the treatment of plants. Patent WO/2009/156688
Lehman LJ, Mccoy RJ, Messenger BJ, Manker DC, Orjala JE, Lindhard D, Marrone PG, Jimenez DR (2000) A strain of Bacillus pumilus for controlling plant diseases. Patent WO/2000/058442
Lehoczky J (1974) Black dead arm disease of grapevine caused by Botryosphaeria stevensii infection. Acta Phytopathol Hung 9:319–327
Leroux P (2003) Modes d’action des produits phytosanitaires sur les organismes pathogènes des plantes. Compt Rend Biol 326:9–21
Leroux P (2004) Chemical control of Botrytis and its resistance to chemical fungicides. In: Elad Y, Williamson B, Tudzynski P, Delen N (eds) Botrytis: biology, pathology and control. Kluwer, Dordrecht, The Nertherlands, pp 195–222
Lingua G, D’Agostino G, Massa N, Antosiano M, Berta G (2002) Mycorrhiza-induced differential response to a yellows disease in tomato. Mycorrhiza 12:191–198
Lo Picollo S, Ferraro V, Alfonzo A, Settanni L, Ercolini D, Burruano S, Moschetti G (2010) Presence of endophytic bacteria in Vitis vinifera leaves as detected by fluorescence in situ hybridization. Ann Microbiol 60:161–167
Loqman S, Barka EA, Clement C, Ouhdouch Y (2009) Antagonistic actinomycetes from Moroccan soil to control the grapevine gray mold. World J Microbiol Biotechnol 25:81–91
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Annu Rev Microbiol 63:541–556
Luque J, Martos S, Phillips AJL (2005) Botryosphaeria viticola sp. nov. on grapevines: a new species with a Dothiorella anamorph. Mycologia 97:1111–1121
Magnin-Robert M, Trotel-Aziz P, Quantinet D, Biagianti S, Aziz A (2007) Biological control of Botrytis cinerea by selected grapevine-associated bacteria and stimulation of chitinase and b-1,3 glucanase activities under field conditions. Eur J Plant Pathol 118:43–57
Maixner M (2011) Recent advances in Bois Noir research. In: 2nd European bois noir workshop 2011. Coop. Libraria Editrice Universita di Padova, Italy, pp 17–32
Marrone PG, Heins SD, Jimenez DR (1999) Methods for controlling above-ground plant diseases using antibiotic-producing Bacillus sp. ATCC 55608 or 55609. US Patent US005869042A
Marshall A (2009) 13.3 million farmers cultivate GM crops. Nature Biotechnol 27:221–221
Mostert L, Halleen F, Fourie P, Crous PW (2006) A review of Phaeoacremonium species involved in Petri disease and esca of grapevines. Phytopathol Mediterr 45:S12–S29
Munkvold GP, Marois JJ (1993) Efficacy of natural epiphytes and colonizers of grapevine pruning wounds for biological control of Eutypa dieback. Phytopathology 83:624–629
Nowak J, Asiedu SK, Lazarovits G, Pillay V, Stewart A, Smith C, Liu Z (1995) Enhancement of in vitro growth and transplant stress tolerance of potato and vegetable plantlets co-cultured with a plant growth-promoting rhizobacterium. In: Carré F, Chagvardieff P (eds) Proceedings of the international symposium on ecophysiology and photosynthetic in vitro cultures. CEA, Aix-en-Provence, France, pp 173–180
Omer AD, Granett J, Wakeman RJ (1999) Pathogenicity of Fusarium oxysporum on different Vitis rootstocks. J Phytopathol 147:433–436
Paul B, Girard I, Bhatnagar T, Bouchet P (1997) Suppression of Botrytis cinerea causing grey mould disease of grape vine (Vitis vinifera) and its pectinolytic activities by a soil bacterium. Microbiol Res 152:413–420
Pearson RC, Goheen AC (eds) (1988) APS. Compendium of grape diseases. APS Press, St Paul, USA
Pezet R, Viret O, Gindro K (2004) Plant-microbe interaction: the Botrytis grey mould of grapes-biology, biochemistry, epidemiology and control management. In: Hemantaranjan A (ed) Advances in plant physiology, vol 7. Scientific publishers, Jodhpur, India, pp 71–116
Phillips AJL (1998) Botryosphaeria dothidea and other fungi associated with excoriose and dieback of grapevines in Portugal. J Phytophatol 146:327–332
Phillips AJL (2002) Botryosphaeria species associated with diseases of grapevines in Portugal. Phytopathol Mediterr 41:3–18
Qin S, Xing K, Jiang J-H, Xu L-H, Li W-J (2011) Biodiversity, bioactive natural products and biotechnological potential of plant-associated endophytic actinobacteria. Appl Microbiol Biotechnol 89:457–473
Ridé M (1996) La nécrose bactérienne de la vigne: données biologiques et épidémiologiques, bases d’une stratégie de lutte. Compt Rend Acad Agri France 82:31–50
Riedle-Bauer M, Sara A, Regner F (2008) Transmission of a stolbur Phytoplasma by the Agalliinae leafhopper Anaceratagallia ribauti (Hemiptera, Auchenorrhyncha, Cicadellidae). J Phytopathol 156:687–690
Riethmueller A, Voglmayr H, Goeker M, Weiss M, Oberwinkler F (2002) Phylogenetic relationships of the downy mildews (Peronosporales) and related groups based on nuclear large subunit ribosomal DNA sequences. Mycologia 94:834–849
Rolshausen PE, Gubler WD (2005) Use of boron for the control of Eutypa dieback of grapevines. Plant Dis 89:734–738
Rolshausen PE, Mahoney NE, Molyneux RJ, Gubler WD (2006) A re-assessment of the species concept in Eutypa lata, the causal agent of Eutypa dieback of grapevine. Phytopathology 96:369–377
Romanazzi G, d’Ascenzo D, Murolo S (2009) Field treatment with resistance inducers for the control of grapevine Bois Noir. J Plant Pathol 91:677–682
Rovesti L, Montermini A (1987) A grapevine decline caused by Sphaeropsis malorum widespread in the province of Reggio-Emilia. Inf Fitopatol 37:59–61
Sawant SD, Sawant IS, Shetty D, Shinde M, Jade S, Waghmare M (2011) Control of powdery mildew in vineyards by Milastin K, a commercial formulation of Bacillus subtilis (KTBS). J Biol Control 25:26–32
Schmidt CS, Wolf GA, Lorenz D, Jaèger J (2001) Biological control of the grapevine dieback fungus Eutypa lata, II.: influence of formulation additives, transposon mutagenesis on the antagonistic activity of Bacillus subtilis, Erwinia herbicola. J Phytopathol 149:437–445
Schoonbeek H-J, Jacquat-Bovet A-C, Mascher F, Métraux J-P (2007) Oxalate-degrading bacteria can protect Arabidopsis thaliana and crop plants against Botrytis cinerea. Mol Plant-Microbe Interact 20:1535–1544
Seemüller E, Harries H (2010) Plant resistance. In: Weintraub PG, Jones P (eds) Phytoplasmas: genomes, plant hosts and vectors. CAB International, Oxfordshire, UK, pp 147–169
Sessitsch A, Coenye T, Sturz AV, Vandamme P, Ait Barka E, Salles JF, van Elsas JD, Faure D, Reiter B, Glick BR, Wang-Pruski G, Nowak J (2005) Burkholderia phytofirmans sp. nov., a novel plant-associated bacterium with plant beneficial properties. Int J Syst Evol Bacteriol 55:1187–1192
Shim JS, Farrand SK, Kerr A (1987) Biological control of crown gall: construction and testing of new biocontrol agents. Phytopathology 77:463–466
Stafford HA (2000) Crown gall disease and Agrobacterium tumefaciens: a study of the history, present knowledge, missing information, and impact on molecular genetics. Bot Rev 66:99–118
Strobel GA, Morrison SL, Cassella M (2005) Protecting plants from oomycete pathogens by treatment with compositions containing Serratamolide and Oocydin A from Serratia marcescens. US Patent US006926892B2
Sule S, Burr TJ (1998) The influence of rootstock resistance to crown gall (Agrobacterium spp.) on the susceptibility of scions in grape vine cultivars. Plant Pathol 47:84–88
Surico G, Mugnai L, Marchi G (2008) The esca disease complex. In: Mukerji KG, Ciancio A (eds) Integrated management of diseases caused by fungi, phytoplasma and bacteria. University of Delhi, Delhi, India, pp 119–136
Svercel M, Christen D, Moënne-Loccoz Y, Duffy B, Défago G (2009) Effect of long-term vineyard monoculture on rhizosphere populations of pseudomonads carrying the antimicrobial biosynthetic genes phlD and/or hcnAB. FEMS Microbiol Ecol 68:25–36
Svercel M, Hamelin J, Duffy B, Moënne-Loccoz Y, Défago G (2010) Distribution of Pseudomonas populations harboring phlD or hcnAB biocontrol genes is related to depth in vineyard soils. Soil Biol Biochem 42:466–472
The Local Monitoring Committee, Lemaire O, Moneyron A, Masson JE (2010) Interactive technology assessment and beyond: the field trial of genetically modified grapevines at INRA-Colmar. PLoS Biol 8:e1000551
Trotel-Aziz P, Aziz A, Magnin-Robert M, Aït Barka E, Gogniès S (2006) Bactéries présentant une activité protectrice de la vigne contre Botrytis cinerea. French patent 06.06.513
Trotel-Aziz P, Couderchet M, Biagianti S, Aziz A (2008) Characterization of new bacterial biocontrol agents Acinetobacter, Bacillus, Pantoea and Pseudomonas spp. mediating grapevine resistance against Botrytis cinerea. Environ Exp Bot 64:21–32
Trouillas FP, Gubler WD (2004) Identification and characterization of Eutypa leptoplaca, a new pathogen of grapevine in Northern California. Mycol Res 108:1195–1204
Trouillas FP, Gubler WD (2010) Pathogenicity of Diatrypaceae species in grapevines in California. Plant Dis 94:867–872
Trouillas FP, Urbez-Torres JR, Gubler WD (2010) Diversity of diatrypaceous fungi associated with grapevine canker diseases in California. Mycologia 102:319–336
Urbez-Torres JR, Gubler WD (2009) Pathogenicity of Botryosphaeriaceae species isolated from grapevine cankers in California. Plant Dis 93:584–592
Úrbez-Torres JR, Peláez H, Santiago Y, Martín C, Moreno C, Gubler WD (2006) Occurrence of Botryosphaeria obtusa, B. dothidea, and B. parva associated with grapevine trunk diseases in Castilla y León region, Spain. Plant Dis 90:835
van der Ent S, van Wees SCM, Pieterse CMJ (2009) Jasmonate signaling in plant interactions with resistance-inducing beneficial microbes. Phytochemistry 70:1581–1588
van Helden M (2008) Protection intégrée: La protection intégrée vis-à-vis des ravageurs de la vigne. In: Kreiter S (ed) Ravageurs de la vigne. Féret, France, pp 321–335
van Loon L (2007) Plant responses to plant growth-promoting rhizobacteria. Eur J Plant Pathol 119:243–254
van Loon LC (2008) Manipulating the plant’s innate immune system by inducing resistance. Phytoparasitica 36:103–106
van Loon LC, Bakker PAHM (2005) Induced systemic resistance as a mechanism of disease suppression by rhizobacteria. In: Siddiqui ZA (ed) PGPR: biocontrol and biofertilization. Springer, Dordrecht, The Netherlands, pp 39–66
van Niekerk JM, Crous PW, Groenewald JZ, Fourie PH, Halleen F (2004) DNA phylogeny, morphology and pathogenicity of Botryosphaeria species on grapevines. Mycologia 96:781–798
van Wees SC, van der Ent S, Pieterse CM (2008) Plant immune responses triggered by beneficial microbes. Curr Opin Plant Biol 11:443–448
Varnier AL, Sanchez L, Vatsa P, Boudesocque L, Garcia-Brugger A, Rabenoelina F, Sorokin A, Renault JH, Kauffmann S, Pugin A, Clément C, Baillieul F, Dorey S (2009) Bacterial rhamnolipids are novel MAMPs conferring resistance to Botrytis cinerea in grapevine. Plant, Cell Environ 32:178–193
Verhagen BWM, Trotel-Aziz P, Couderchet M, Höfte M, Aziz A (2010) Pseudomonas spp.-induced systemic resistance to Botrytis cinerea is associated with induction and priming of defence responses in grapevine. J Exp Bot 61:249–260
Verhagen BWM, Trotel-Aziz P, Jeandet P, Baillieul F, Aziz A (2011) Improved resistance against Botrytis cinerea by grapevine-associated bacteria that induce a prime oxidative burst and phytoalexin Production. Phytopathology 101:768–777
Vidal JR, Kikkert JR, Malnoy MA, Wallace PG, Barnard J, Reisch BI (2006) Evaluation of transgenic ‘Chardonnay’ (Vitis vinifera) containing magainin genes for resistance to crown gall and powdery mildew. Trans Res 15:69–82
Wei Q, Li J-Y, Wang J-H, Wang H-M (2009) Strain E26 of Agrobacterium vitis, a biological control agent of grapevine crown gall, does not contain virA and virG pathogenic determinants. J Phytopathol 157:657–665
Welbaum G, Sturz AV, Dong Z, Nowak J (2004) Fertilizing soil microorganisms to improve productivity of agroecosystems. Crit Rev Plant Sci 23:175–193
Zhang L, Zhang G, Qian X, Li G (2009) First report of Verticillium wilt of grapevine (Vitis vinifera) caused by Verticillium dahliae in China. Plant Dis 93:841
Zhang S, Flores CZ, Kumar D, Chakrabarty P, Hopkins DL, Gabriel DW (2011) The Xylella fastidiosa biocontrol strain EB92-1 genome is very similar and syntenic to Pierce’s disease strains. J Bacteriol 193:5576–5577
Ziedan E-SH, El-Mohamedy RSR (2008) Application of Pseudomonas fluorescens for controlling root-rot disease of grapevine. Res J Agric Biol Sci 4:346–353
Ziedan ESH, Farrag ES, El-Mohamedy RS, Abd Alla MA (2010) Streptomyces alni as a biocontrol agent to root-rot of grapevine and increasing their efficiency by biofertilisers inocula. Arch Phytopathol Plant Prot 43:634–646
Ziedan E-SH, Embaby E-SM, Farrag ES (2011) First record of Fusarium vascular wilt on grapevine in Egypt. Arch Phytopathol Plant Prot 44:1719–1727
Acknowledgments
This work was supported by a BQR program from National Polytechnic Institute of Toulouse, France, regional projects in Midi-Pyrénées, a “FUI” project from Ministry of France as well as by High Education Program from Pakistan that help us to work on biocontrol of plant diseases and in particular on grapevine infections, beneficial microbes and their metabolites of interest. We also acknowledge cost action FA1103 for helping cooperation and we are grateful to Anne Alibert (INP-ENSAT, France) for reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Stéphane Compant, Günter Brader, and Saima Muzammil contributed equally to this work.
Handling Editor: Jesus Mercado Blanco
Rights and permissions
About this article
Cite this article
Compant, S., Brader, G., Muzammil, S. et al. Use of beneficial bacteria and their secondary metabolites to control grapevine pathogen diseases. BioControl 58, 435–455 (2013). https://doi.org/10.1007/s10526-012-9479-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-012-9479-6