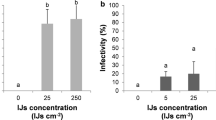
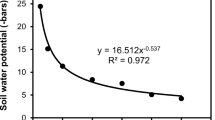
Abstract
To investigate nematode establishment and persistence, dauer juveniles (DJs) of Heterorhabditis bacteriophora were applied at 50 cm-2 in different crops in June and July with conventional spraying equipment and 420 l water ha-1. Application hardly had any effects on survival and infectivity. The number of DJs reaching the soil was assessed and the establishment and persistence recorded by baiting soil samples with larvae of the wax moth Galleria mellonella. The better the plant canopy was developed the fewer DJs reached the soil during application. Whereas in pasture 77% and in potatoes 78% of the applied nematodes reached the soil, in wheat and peas little less than 50%, in oil-seed rape only 5% and in lupine 6% were recorded. Between 50 and 60% of the soil samples contained H. bacteriophora a month after application with the exception of wheat (>90%) and potatoes (<5%) indicating that the number of nematodes reaching the soil during application had no influence on their establishment in the soil. Probably DJs can survive in the plant canopy and reach the soil later after application. The percentage of nematode-positive soil samples dropped considerably after tillage. In potatoes no nematodes were recovered after two months, which probably was also due to the intensive movement of the soil. Although nematodes are susceptible to freezing, temperatures below 0°C during the winter did not extinguish the H. bacteriophora population. In field crops EPN usually persisted not much longer than one year. The longest persistence of H. bacteriophora was detected 23 months after release in beans followed in rotation by wheat with red clover as cover crop. In this field larvae of the pea weevil Sitona lineatus (Coleoptera: Curculionidae) were detected in soil samples and found infected with the released nematode population. In the laboratory the field soils were tested for persistence of H. bacteriophora at 8°C and a half-life of 24.8 days was recorded in the absence of host insects and plants. Thus long-term persistence in the field was a result of recycling in host insects, which could not be detected in other crops than beans and clover. As H. bacteriophora seems to be restricted in its host potential, this species disappears after release once the host population is not available anymore.



Similar content being viewed by others
References
Abbott WS (1925) A method for computing the effectiveness of an insecticide. J Econ Entomol 18:265–267
Akhurst RJ, Bedding RA, Bull R-M, Smith RJ (1992) An epizootic of Heterorhabditis spp. (Heterorhabditidae: Nematoda) in sugar cane scarabaeida (Coleoptera). Fundam Appl Nematol 15:71–73
Barbercheck ME, Kaya HK (1991) Effect of host condition and soil texture on host finding by the entomogenous nematodes Heterorhabditis bacteriophora (Rhabditida: Heterorhabditidae) and Steinernema carpocapsae (Rhabditida: Steinernematidae). Environ Entomol 20:582–589
Baur ME, Kaya K (2001) Persistence of entomopathogenic nematodes. In: Population of Entomopathogenic Nematodes in Foodwebs In: Gaugler R (ed) Entomopathogenic Nematology. CABI Publishing, UK, pp 225–240
Bedding RA, Akhurst RJ (1975) A simple technique for the detection of insect parasitic nematodes in soil. Nematologica 21:109–110
Brust GE (1991) Augmentation of an endemic entomogenous nematode by agroecosystem manipulation for the control of a soil pest. Agric Ecosyst Environ 36:175–184
Chapple AC, Taylor RAJ, Hall FR (1995) The transformation of spatially determined drop sizes to their temporal equivalents for agricultural sprays. J Agric Eng Res 60:49–56
Ehlers R-U (1998) Entomopathogenic nematodes – save biocontrol agents for sustainable systems. Phytoprotection 79:94–102
Ehlers R-U (2001) Mass production of entomopathogenic nematodes for plant protection. Appl Microbiol Biotechnol 56:623–633
Ehlers R-U (2003) Biocontrol Nematodes. In: Hokkanen HMT, Hajek AE (eds) Environmental Impacts of Microbial Insecticides – Need and Methods for Risk Assessment. Kluwer Academic Publisher, Dordrecht, pp 177–220
Eichhorn O (1988) Untersuchungen über die Fichtengespinstblattwespen Cephalcia spp. PANZ. (Hymenoptera, Pamphiliidae) II. Die Larven- und Nymphenparasiten. J Appl Entomol 105:105–140
Epsky ND, Walter DE, Capinerea JL (1988) Potential role of microarthropods as biotic mortality factors of entomogenous nematodes (Rhabditidae: Steinernematidae, Heterorhabditidae). J Econ Entomol 81:821–825
Finney DJ (1971) Probit Analysis. Cambridge University, London, England, UK
Fitters PFL, Griffin CT (2006) Survival, starvation, and activity in Heterorhabditis megidis (Nematoda: Heterorhabditidae). Biol Control 37:82–88
Forschler BT, Gardner WA (1991) Field efficacy and persistence of entomogenous nematodes in the management of white grubs (Coleoptera: Scarabaeidae) in turf and pasture. J Econ Entomol 84:1454–1459
Forst S, Clarke D (2002) Bacteria-nematode symbiosis. In: Gaugler R (ed) Entomopathogenic Nematology. CABI Publishing, Oxon, UK, pp 57–77
Gaugler R, Bednarek A, Campbell JF (1992) Ultraviolet inactivation of heterorhabditid and steinernematid nematodes. J Invertebrate Pathol 59:155–160
Gilmore SK, Potter DA (1993) Potential role of Collembola as biotic mortality agents for entomopathogenic nematodes. Pedobiologia 37:30–38
Glazer I (2002) Survival Biology. In: Gaugler R (ed) Entomopathogenic Nematology. CABI Publishing, Oxon, UK, pp 169–187
Grewal PS, Ehlers R-U, Shapiro-Ilan DI (2006) Nematodes as Biocontrol Agents, CABI Publishing, Oxon, UK
Grewal PS, Selvan S, Gaugler R (1994) Thermal adaptation of entomopathogenic nematodes: niche breadth for infection, establishment, and reproduction. J Thermal Biol 19:245–253
Griffin CT (1993) Temperature responses of entomopathogenic nematodes: Implications for the success of biological control programmes. In: Bedding RAR, Kaya H (eds) Nematodes and the biological control of insect pests. CSIRO, East Melbourne, pp 115–126
Hominick WM (2002) Biogeography. In: Gaugler R (ed) Entomopathogenic Nematology. CABI Publishing, Oxon, UK, pp 115–143
Hsiao W, All JN (1998) Effects of temperature and placement site on the dispersal of the entomopathogenic nematode, Steinernema carpocapsae in four soils. Chin J Entomol 16:95–106
Hummel RL, Walgenbach JF, Barbercheck ME, Kennedy GG, Hoyt GD, Arellano C (2002) Effects of production practices on soil-borne entomopathogens in Western North Carolina vegetable systems. Environ Entomol 31:84–91
Kaya HK (1990) Soil ecology. In: Gaugler R, Kaya HK (eds) Entomopathogenic nematodes in biological control. CRC Press, Boca Raton, pp 93–115
Kaya HK, Thurston GS (1993) Soil microorganisms affecting entomopathogenic nematodes. In: Bedding R, Akhurst RR, Kaya H (eds) Nematodes and the biological control of insect pests. CSIRO, East Melbourne, pp 97–104
Kaya HK, Stock P (1997) Techniques in insect nematology. In: Lacey LA (eds) Manual of techniques in insect pathology. Academic Press, San Diego, pp 282–324
Klein MG, Georgis R (1992) Persistence of control of Japanese beetle (Coleoptera: Scarabaeidae) larvae with steinernematid and heterorhabditid nematodes. J Econ Entomol 85:727–730
Koppenhöfer AM, Jaffee BA, Muldoon AE, Strong DR, Kaya HK (1996) Effect of nematode-trapping fungi on an entomopathogenic nematode originating from the same field site in California. J Invertebrate Pathol 68:246–252
Kung SP, Gaugler R, Kaya HK (1990a) Soil type and entomopathogenic nematode persistence. J Invertebrate Pathol 55:401–406
Kung SP, Gaugler R, Kaya HK (1990b) Influence of soil pH and oxygen on persistence of Steinernema spp. J Nematol 22:440–445
Kung SP, Gaugler R, Kaya HK (1991) Effects of soil temperature, moisture, and relative humidity on entomopathogenic nematode persistence. J Invertebrate Pathol 57:242–249
Laws RM, Franks F (1990) Life at low temperatures. The Royal Society, London
Millar LC, Barbercheck ME (2002) Effects of tillage practices on entomopathogenic nematodes in a corn agroecosystem. Biol Control 25:1–11
Molyneux AS (1985) Survival of infective juveniles of Heterorhabditis spp. and Steinernema spp. (Nematoda: Rhabditida) at various temperatures and their subsequent infectivity for insects. Revue de Nématolgie 8:165–170
Mrácek Z (1986) Nematodes and other factors controlling the sawfly Cephalcia abietes (Pamphilidae: Hymenoptera), in Czechoslovakia. For Ecol Manage 15:75–79
Schroer S, Ehlers R-U (2005a) Foliar application of the entomopathogenic nematode Steinernema carpocapsae for biological control of diamondback moth larvae (Plutella xylostella). Biol Control 33:81–86
Schroer S, Ziermann D, Ehlers R-U (2005b) Mode of action of a surfactant-polymer-formulation to support performance of the entomopathogenic nematode Steinernema carpocapsae for control of diamondback moth larvae (Plutella xylostella). Bioc Sci Technol 15:601–613
Shapiro-Ilan DI, Gougle DH, Koppenhöfer AM (2002) Factors affecting commercial success: Case studies in cotton, turf and citrus. In: Gaugler R (ed) Entomopathogenic Nematology. CABI Publishing, Oxon, UK, pp 333–355
Shapiro DI, Tylka GL, Lewis LC (1996) Effects of fertilizers on virulence of Steinernema carpocapsae. Appl Soil Ecol 3:27–34
Smits PH (1996) Post-application persistence of entomopathogenic nematodes. Bioc Sci Technol 6:379–387
Statistica (1991) Complete Statistical System by StatSoft, Inc. 2325 East 13th Street, Tulsa, OK 74104
Stirling GR (1988) Biological control of plant-parasitic nematodes. In: Poinar GOJ, Jansson HB (eds) Diseases of nematodes, vol 2. CRC Press Inc., Boca Raton, Florida, USA, pp 93–139
Strong DR (2002) Population of entomopathogenic nematodes in foodwebs. In: Gaugler R (ed) Entomopathogenic Nematology. CABI Publishing, Oxon, UK, pp 225–240
Sturhan D (1996) Prevalence and habitat specificity of entomopathogenic nematodes in Germany. COST –819 Application and persistence of entomopathogenic nematodes, Proceeding of a workshop held at Todi, Perugia, Italy 19–20 May 1995
Sturhan D, Mracek Z (2000) Comparison of the Galleria baiting technique and a direct extraction method for recovering Steinernema (Nematoda: Rhabditida) infective-stage juveniles from soil. Folia Parasitologica 47:315–318
Sulistyanto D, Ehlers R-U (1996) Efficacy of the entomopathogenic nematodes Heterorhabditis megidis and Heterorhabditis bacteriophora for the control of grubs (Phyllopertha horticola and Aphodius contaminatus) in golf course turf. Bioc Sci Technol 6:247–250
Susurluk A, Dix I, Stackebrandt E, Strauch O, Wyss U, Ehlers R-U (2001) Identification and ecological characterisation of three entomopathogenic nematode-bacterium complexes from Turkey. Nematology 3:833–841
Toepfer S, Gueldenzoph C, Ehlers R-U, Kuhlmann U (2005) Screening of entomopathogenic nematodes for virulence against the invasive western corn rootworm, Diabrotica virgifera virgifera (Coleoptera: Chrysomelidae) in Europe. Bull Entomol Res 95:473–482
Walter DE (1987) Life history, trophic behavior, and description of Gamasellodes vermivorax n.sp (Mesostigmata: Ascidae), a predator of nematodes and arthropods in semiarid grassland soils. Can J Zool 65:1689–1695
Walter DE (1988) Predation and mycophagy by endeostigmatid mites (Acariformes: Prostigmata). Exp Appl Acarol 4:159–166
Wilson M, Gaugler R (2003) Factors limiting short term persistence of entomopathogenic nematodes. J Appl Entomol 128:250–253
Wright DJ, Peters A, Schroer S, Fife JP (2005) Application technology. In: Grewal PS, Ehlers R-U, Shapiro-Ilan DI (eds) Nematodes as biological control agenst. CABI Publishing, Oxfordshire, UK, pp 91–106
Acknowledgements
Thanks are due to e-nema GmbH for a scholarship to the first author and to Joachim Postel for proving his fields for experiments. We also thank Johanna Schmidt, Doris Ziermann, Michael Wingen, Helga Ladehoff, Xiaoli Yi and Nicola Benecke for technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Susurluk, A., Ehlers, RU. Field persistence of the entomopathogenic nematode Heterorhabditis bacteriophora in different crops. BioControl 53, 627–641 (2008). https://doi.org/10.1007/s10526-007-9104-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-007-9104-2