Abstract
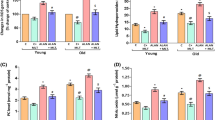
Aging is one of the main risk factors for cardiovascular diseases, and oxidative stress is a key element responsible for the development of age-related pathologies. In addition, the alteration of circadian rhythms also contributes to cardiovascular pathology, but the underlying mechanisms are not well defined. We investigated the aging consequences on the temporal patterns of antioxidant defenses, the molecular clock machinery, and the blood pressure, in the heart of male rats maintained under constant darkness (free running) conditions. Male Holtzman rats from young adult (3-month-old) and older (22-month-old) groups were maintained under constant darkness (12-h dark:12-h dark, DD) condition during fifteen days before the experiment. After the DD period, heart ventricle samples were isolated every 4-h throughout a 24-h period. We observed circadian rhythms of catalase (CAT) and glutathione peroxidase (GPx) mRNA expression, as well as ultradian rhythms of Nrf2 mRNA levels, in the heart of young adult rats. We also found circadian oscillations of CAT and GPx enzymatic activities, reduced glutathione (GSH) and BMAL1 protein in the same group. Interestingly, aging abolished the rhythms of CAT and GPx enzymatic activities, phase-shifted the rhythm’s acrophases of GSH and BMAL1 protein levels and turned circadian the ultradian oscillation of Nrf2 expression. Moreover, aging phase-shifted the circadian pattern of systolic blood pressure. In conclusion, aging modifies the temporal organization of antioxidant defenses and blood pressure, probably, as a consequence of a disruption in the circadian rhythm of the clock’s transcriptional regulator, BMAL1, in heart.







Similar content being viewed by others
Data availability
The data that support the findings of the current study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/s0076-6879(84)05016-3
Akerboom TP, Sies H (1981) Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol 77:373–382. https://doi.org/10.1016/s0076-6879(81)77050-2
Albrecht U (2012) Timing to perfection: the biology of central and peripheral circadian clocks. Neuron 74:246–260. https://doi.org/10.1016/j.neuron.2012.04.006
Anea CB, Zhang M, Stepp DW, Simkins GB, Reed G, Fulton DJ, Rudic RD (2009) Vascular disease in mice with a dysfunctional circadian clock. Circulation 119:1510–1517. https://doi.org/10.1161/CIRCULATIONAHA.108.827477
Aschoff J (1967) Human circadian rhythms in activity, body temperature and other functions. Life Sci Space Res 5:159–173
Balaban RS, Nemoto S, Finke T (2005) Mitochondria, oxidants, and aging. Cell 120:483–449. https://doi.org/10.1016/j.cell.2005.02.001
Bollinger T, Schibler U (2014) Circadian rhythms—from genes to physiology and disease. Swiss Med Wkly 144:w13984. https://doi.org/10.4414/smw.2014.13984
Bonaconsa M, Malpeli G, Montaruli A, Carandente F, Grassi-Zucconi G, Bentivoglio M (2014) Differential modulation of clock gene expression in the suprachiasmatic nucleus, liver and heart of aged mice. Exp Gerontol 55:70–79. https://doi.org/10.1016/j.exger.2014.03.011
Bryan HK, Olayanju A, Goldring CE, Park BK (2013) The Nrf2 cell defense pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem Pharmacol 85:705–717. https://doi.org/10.1016/j.bcp.2012.11.016
Buhr ED, Takahashi JS (2013) Molecular components of the mammalian circadian clock. Handb Exp Pharmacol 217:3–27. https://doi.org/10.1007/978-3-642-25950-0_1
Chen QM, Maltagliati AJ (2018) Nrf2 at the heart of oxidative stress and cardiac protection. Physiol Genomics 50:77–97. https://doi.org/10.1152/physiolgenomics.00041.2017
Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS, Fitzgerald GA (2007) Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci USA 104:3450–3455. https://doi.org/10.1073/pnas.0611680104
Dashti HS, Aslibekyan S, Scheer FA, Smith CE, Lamon-Fava S, Jacques P, Lai CQ, Tucker KL, Arnett DK, Ordovás JM (2016) Clock genes explain a large proportion of phenotypic variance in systolic blood pressure and this control is not modified by environmental temperature. Am J Hypertens 29:132–140. https://doi.org/10.1093/ajh/hpv082
Dibner C, Schibler U, Albrecht U (2010) The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol 72:517–549. https://doi.org/10.1146/annurev-physiol-021909-135821
Douma LG, Gumz ML (2018) Circadian clock-mediated regulation of blood pressure. Free Radic Biol Med 119:108–114. https://doi.org/10.1016/j.freeradbiomed.2017.11.024
Elavarasan J, Velusamy P, Ganesan T, Ramakrishnan SK, Rajasekaran D, Periandavan K (2012) Hesperidin-mediated expression of Nrf2 and upregulation of antioxidant status in senescent rat heart. J Pharm Pharmacol 64:1472–1482. https://doi.org/10.1111/j.2042-7158.2012.01512.x
Flohé L, Günzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105:114–121. https://doi.org/10.1016/s0076-6879(84)05015-1
Fonzo LS, Golini RS, Delgado SM, Ponce IT, Bonomi MR, Rezza IG, Giménez MS, Anzulovich AC (2009) Temporal patterns of lipoperoxidation and antioxidant enzymes are modified in the hippocampus of vitamin A-deficient rats. Hippocampus 19:869–880. https://doi.org/10.1002/hipo.20571
Foster RG, Hughes S, Peirson SN (2020) Circadian Photoentrainment in Mice and Humans. Biology (basel) 9:180. https://doi.org/10.3390/biology9070180
Golombek DA, Rosenstein RE (2010) Physiology of circadian entrainment. Physiol Rev 90:1063–1102. https://doi.org/10.1152/physrev.00009.2009
Goodwin J, Pearce VR, Taylor RS, Read KL, Powers SJ (2001) Seasonal cold and circadian changes in blood pressure and physical activity in young and elderly people. Age Ageing 30:311–317. https://doi.org/10.1093/ageing/30.4.311
Hardeland R, Coto-Montes A, Poeggeler B (2003) Circadian rhythms, oxidative stress, and antioxidative defense mechanisms. Chronobiol Int 20:921–962. https://doi.org/10.1081/CBI-120025245
Ingle KA, Kain V, Goel M, Prabhu SD, Young ME, Halade GV (2015) Cardiomyocyte-specific Bmal1 deletion in mice triggers diastolic dysfunction, extracellular matrix response, and impaired resolution of inflammation. Am J Physiol Heart Circ Physiol 309:H1827-1836. https://doi.org/10.1152/ajpheart.00608.2015
Izzo C, Vitillo P, Di Pietro P, Visco V, Strianese A, Virtuoso N, Ciccarelli M, Galasso G, Carrizzo A, Vecchione C (2021) The role of oxidative stress in cardiovascular aging and cardiovascular diseases. Life (Basel) 11:60. https://doi.org/10.3390/life11010060
Jud C, Schmutz I, Hampp G, Oster H, Albrecht U (2005) A guideline for analyzing circadian wheel-running behavior in rodents under different lighting conditions. Biol Proced Online 7:101–116. https://doi.org/10.1251/bpo109
Lacoste MG, Ponce I, Golini R, Delgado S, Anzulovich AC (2017) Aging modifies daily variation of antioxidant enzymes and oxidative status in the hippocampus. Exp Gerontol 88:42–50. https://doi.org/10.1016/j.exger.2016.12.002
MacNee W, Rabinovich RA, Choudhury G (2014) Ageing and the border between health and disease. Eur Respir J 44:1332–1352. https://doi.org/10.1183/09031936.00134014
Maher P (2005) The effects of stress and aging on glutathione metabolism. Ageing Res Rev 4:288–314. https://doi.org/10.1016/j.arr.2005.02.005
Manikonda PK, Jagota A (2012) Melatonin administration differentially affects age induced alterations in daily rhythms of lipid peroxidation and antioxidant enzymes in male rat liver. Biogerontology 13:511–524. https://doi.org/10.1007/s10522-012-9396-1
Martino TA, Young ME (2015) Influence of the cardiomyocyte circadian clock on cardiac physiology and pathophysiology. J Biol Rhythms 30:183–205. https://doi.org/10.1177/0748730415575246
Merbitz-Zahradnik T, Wolf E (2015) How is the inner circadian clock controlled by interactive clock proteins? Structural analysis of clock proteins elucidates their physiological role. FEBS Lett 589:1516–1529. https://doi.org/10.1016/j.febslet.2015.05.024
Mikhed Y, Daiber A, Steven S (2015) Mitochondrial oxidative stress, mitochondrial DNA damage and their role in age-related vascular dysfunction. Int J Mol Sci 16:15918–15953. https://doi.org/10.3390/ijms160715918
Millar-Craig MW, Bishop CN, Raftery EB (1978) Circadian variation of blood-pressure. Lancet 1:795–797. https://doi.org/10.1016/s0140-6736(78)92998-7
Moore-Ede MC (1986) Physiology of the circadian timing system: predictive versus reactive homeostasis. Am J Physiol 250:R737–R752. https://doi.org/10.1152/ajpregu.1986.250.5.R737
Nakahata Y, Yoshida M, Takano A, Soma H, Yamamoto T, Yasuda A, Nakatsu T, Takumi T (2008) A direct repeat of E-box-like elements is required for cell-autonomous circadian rhythm of clock genes. BMC Mol Biol 9:1. https://doi.org/10.1186/1471-2199-9-1
Navigatore-Fonzo LS, Delgado SM, Golini RS, Anzulovich AC (2014) Circadian rhythms of locomotor activity and hippocampal clock genes expression are dampened in vitamin A-deficient rats. Nutr Res 34:326–335. https://doi.org/10.1016/j.nutres.2014.02.002
North BJ, Sinclair DA (2012) The intersection between aging and cardiovascular disease. Circ Res 110:1097–1108. https://doi.org/10.1161/CIRCRESAHA.111.246876
Patel SA, Velingkaar NS, Kondratov RV (2014) Transcriptional control of antioxidant defense by the circadian clock. Antioxid Redox Signal 20:2997–3006. https://doi.org/10.1089/ars.2013.5671
Pekovic-Vaughan V, Gibbs J, Yoshitane H, Yang N, Pathiranage D, Guo B, Sagami A, Taguchi K, Bechtold D, Loudon A, Yamamoto M, Chan J, van der Horst GT, Fukada Y, Meng QJ (2014) The circadian clock regulates rhythmic activation of the NRF2/glutathione-mediated antioxidant defense pathway to modulate pulmonary fibrosis. Genes Dev 28:548–560. https://doi.org/10.1101/gad.237081.113
Ponce IT, Rezza IG, Delgado SM, Navigatore LS, Bonomi MR, Golini RL, Giménez MS, Anzulovich AC (2012) Daily oscillation of glutathione redox cycle is dampened in the nutritional vitamin A deficiency. Biol Rhythm Res 43:351–372. https://doi.org/10.1080/09291016.2011.593847
Quandt K, Frech KK, Karas H, Wingender E, Werner T (1995) MatInd and MatInspector: new fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res 23:4878–4884. https://doi.org/10.1093/nar/23.23.4878
Refinetti R, Lissen GC, Halberg F (2007) Procedures for numerical analysis of circadian rhythms. Biol Rhythm Res 38:275–325. https://doi.org/10.1080/09291010600903692
Richards J, Diaz AN, Gumz ML (2014) Clock genes in hypertension: novel insights from rodent models. Blood Press Monit 19:249–254. https://doi.org/10.1097/MBP.0000000000000060
Rodrigo GC, Herbert KE (2018) Regulation of vascular function and blood pressure by circadian variation in redox signalling. Free Radic Biol Med 1(119):115–120. https://doi.org/10.1016/j.freeradbiomed.2017.10.381
Rutter J, Reick M, Wu LC, McKnight SL (2001) Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science 293:510–514. https://doi.org/10.1126/science.1060698
Sahoo S, Meijles DN, Pagano PJ (2016) NADPH oxidases: key modulators in aging and age-related cardiovascular diseases? Clin Sci 130:317–335. https://doi.org/10.1042/CS20150087
Sano H, Hayashi H, Makino M, Takezawa H, Hirai M, Saito H, Ebihara S (1995) Effects of suprachiasmatic lesions on circadian rhythms of blood pressure, heart rate and locomotor activity in the rat. Jpn Circ J 59:565–573. https://doi.org/10.1253/jcj.59.565
Schriner S, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS (2005) Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 308:1909–1911. https://doi.org/10.1126/science.1106653
Shih PH, Yen GC (2007) Differential expressions of antioxidant status in aging rats: the role of transcriptional factor Nrf2 and MAPK signaling pathway. Biogerontology 8:71–80. https://doi.org/10.1007/s10522-006-9033-y
Stewart J, Manmathan G, Wilkinson P (2017) Primary prevention of cardiovascular disease: a review of contemporary guidance and literature. JRSM Cardiovasc Dis 6:2048004016687211. https://doi.org/10.1177/2048004016687211
Thaela MJ, Jensen MS, Cornélissen G, Halberg F, Nöddegaard F, Jakobsen K, Pierzynowski SG (1998) Circadian and ultradian variation in pancreatic secretion of meal-fed pigs after weaning. J Anim Sci 76:1131–1139. https://doi.org/10.2527/1998.7641131x
Ungvari Z, Bailey-Downs L, Sosnowska D, Gautam T, Koncz P, Losonczy G, Ballabh P, de Cabo R, Sonntag WE, Csiszar A (2011) Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of NRF2-mediated antioxidant response. Am J Physiol Heart Circ Physiol 301:H363-372. https://doi.org/10.1152/ajpheart.01134.2010
Viña J, Borras C, Abdelaziz KM, Garcia-Valles R, Gomez-Cabrera MC (2013) The free radical theory of aging revisited: the cell signaling disruption theory of aging. Antioxid Redox Signal 19:779–787. https://doi.org/10.1089/ars.2012.5111
Wible RS, Ramanathan C, Sutter CH, Olesen KM, Kensler TW, Liu AC, Sutter TR (2018) NRF2 regulates core and stabilizing circadian clock loops, coupling redox and timekeeping in Mus musculus. eLfe. https://doi.org/10.7554/eLife.31656
Xie Z, Su W, Liu S, Zhao G, Esser K, Schroder EA, Lefta M, Stauss HM, Guo Z, Gong MC (2015) Smooth-muscle BMAL1 participates in blood pressure circadian rhythm regulation. J Clin Invest 125:324–336. https://doi.org/10.1172/JCI76881
Xu YQ, Zhang D, Jin T, Cai DJ, Wu Q, Lu Y, Liu J, Klaassen CD (2012) Diurnal variation of hepatic antioxidant gene expression in mice. PLoS ONE 7:e44237. https://doi.org/10.1371/journal.pone.0044237
Xue M, Momiji H, Rabbani N, Barker G, Bretschneider T, Shmygol A, Rand DA, Thornalley PJ (2015) Frequency modulated translocational oscillations of Nrf2 mediate the antioxidant response element cytoprotective transcriptional response. Antioxid Redox Signal 23:613–629. https://doi.org/10.1089/ars.2014.5962
Wu G, Fang YZ, Yang S, Lupton JR, Turner ND (2004) Glutathione metabolism and its implications for health. J Nutr 134:489–492. https://doi.org/10.1093/jn/134.3.489
Young ME, Brewer RA, Peliciari-Garcia RA, Collins HE, He L, Birky TL, Peden BW, Thompson EG, Ammons BJ, Bray MS, Chatham JC, Wende AR, Yang Q, Chow CW, Martino TA, Gamble KL (2014) Cardiomyocyte-specific BMAL1 plays critical roles in metabolism, signaling, and maintenance of contractile function of the heart. J Biol Rhythms 29:257–276. https://doi.org/10.1177/0748730414543141
Zachariah PK, Cornelissen G, Halberg F (1990) Ambulatory cardiovascular monitoring of healthy adults in Rochester, Minnesota: chronobiologic assessment. Prog Clin Biol Res 341A:243–254
Zhang YK, Yeager RL, Klaassen CD (2009) Circadian expression profiles of drug-processing genes and transcription factors in mouse liver. Drug Metab Dispos 37:106–115. https://doi.org/10.1124/dmd.108.024174
Zhang H, Davies KJ, Forman HJ (2015) Oxidative stress response and Nrf2 signaling in aging. Free Radic Biol Med 88:314–336. https://doi.org/10.1016/j.freeradbiomed.2015.05.036
Zhu H, Itoh K, Yamamoto M, Zweier JL, Li Y (2005) Role of Nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: protection against reactive oxygen and nitrogen species-induced cell injury. FEBS Lett 579:3029–3036. https://doi.org/10.1016/j.febslet.2005.04.058
Zuther P, Gorbey S, Lemmer B (2009) Chronos-Fit 1.06, http://www.ma.uni-heidelberg.de/inst/phar/lehre/chrono.html
Acknowledgements
We acknowledge the Laboratorio de Cronobiología at the Instituto Multidisciplinario de Investigaciones Biológicas-San Luis (IMIBIO-SL), the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and the Facultad de Química, Bioquímica y Farmacia (FQByF) at the Universidad Nacional de San Luis (UNSL). Maria G Lacoste and Ana C Anzulovich are members of the research career at the CONICET.
Funding
This work was supported by the Agencia Nacional de Promoción Científica y Tecnológica [BID Grant PICT 2016-0332-FONCyT-ANPCyT, Argentina], the Consejo Nacional de Investigaciones Científicas y Técnicas [Grant PIP 00446-CONICET, Argentina] and the Universidad Nacional de San Luis [Grant PROICO 2-0518-UNSL, Argentina].
Author information
Authors and Affiliations
Contributions
ACA, MGL and SMD contributed to the study conception and design. FGA, ICP and MLT performed the experiments and/or data collection. FGA, MLF, MGL and ACA analyzed and interpreted data. The first draft of the manuscript was written by FGA and MGL and all authors contributed, revised critically, and commented on the manuscript. ACA: funding acquisition and supervision. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted (National University of San Luis Committee's Guidelines for the Care and Use of Experimental Animals, Protocols Nos. B-83/16 and B-242/16 approved by Res. RCD-2–61/17-UNSL and Protocols Nos. B-83/17, B-278/17 and B-279/17 approved by Res. RCD-2–296/17-UNSL).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Altamirano, F.G., Castro-Pascual, I.C., Ferramola, M.L. et al. Aging disrupts the temporal organization of antioxidant defenses in the heart of male rats and phase shifts circadian rhythms of systolic blood pressure. Biogerontology 22, 603–621 (2021). https://doi.org/10.1007/s10522-021-09938-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-021-09938-7