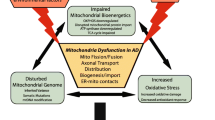
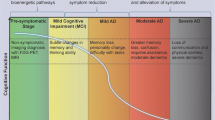
Abstract
After more than 80 years from the revolutionary discoveries of Otto Warburg, who observed high glucose dependency, with increased glycolysis and lactate production regardless of oxygen availability in most cancer cells, the ‘Warburg effect’ returns to the fore in neuronal cells affected by Alzheimer’s disease (AD). Indeed, it seems that, in the mild phase of AD, neuronal cells “prefer” to use the energetically inefficient method of burning glucose by glycolysis, as in cancer, proving to become resistant to β-amyloid (Aβ)-dependent apoptosis. However, in the late phase, while most AD brain cells die in response to Aβ toxicity, only small populations of neurons, exhibiting increased glucose uptake and glycolytic flux, are able to survive as they are resistant to Aβ. Here we draw an overview on the metabolic shift for glucose utilization from oxidative phosphorylation to glycolysis, focusing on the hypothesis that, as extreme attempt to oppose the impending death, mitochondria—whose dysfunction and central role in Aβ toxicity is an AD hallmark—are sent into quiescence, this likely contributing to activate mechanisms of resistance to Aβ-dependent apoptosis. Finally, the attempt turns out fruitless since the loss of the adaptive advantage afforded by elevated aerobic glycolysis exacerbates the pathophysiological processes associated with AD, making the brain susceptible to Aβ-induced neurotoxicity and leading to cell death and dementia. The understanding of how certain nerve cells become resistant to Aβ toxicity, while the majority dies, is an attractive challenge toward the identification of novel possible targets for AD therapy.

Similar content being viewed by others
References
Abu-Hamad S, Arbel N, Calo D, Arzoine L, Israelson A, Keinan N, Ben-Romano R, Friedman O, Shoshan-Barmatz V (2009) The VDAC1 N-terminus is essential both for apoptosis and the protective effect of antiapoptotic proteins. J Cell Sci 122:1906–1916
Amadoro G, Corsetti V, Ciotti MT, Florenzano F, Capsoni S, Amato G, Calissano P (2011) Endogenous Aβ causes cell death via early tau hyperphosphorylation. Neurobiol Aging 32:969–990
Amadoro G, Corsetti V, Atlante A, Florenzano F, Capsoni S, Bussani R, Mercanti D, Calissano P (2012) Interaction between NH(2)-tau fragment and Aβ in Alzheimer’s disease mitochondria contributes to the synaptic deterioration. Neurobiol Aging 33:833.e1-25
Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA (2004) Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol 61:661–666
Ashraf A, Fan Z, Brooks DJ, Edison P (2015) Cortical hypermetabolism in MCI subjects: a compensatory mechanism? Eur J Nucl Med Mol Imaging 42:447–458
Atlante A, Gagliardi S, Minervini GM, Marra E, Passarella S, Calissano P (1996) Rapid uncoupling of oxidative phosphorylation accompanies glutamate toxicity in rat cerebellar granule cells. Neuroreport 7:2519–2523
Atlante A, Bobba A, Calissano P, Passarella S, Marra E (2003) The apoptosis/necrosis transition in cerebellar granule cells depends on the mutual relationship of the antioxidant and the proteolytic systems which regulate ROS production and cytochrome c release en route to death. J Neurochem 84:960–971
Atlante A, Giannattasio S, Bobba A, Gagliardi S, Petragallo V, Calissano P, Marra E, Passarella S (2005) An increase in the ATP levels occurs in cerebellar granule cells en route to apoptosis in which ATP derives from both oxidative phosphorylation and anaerobic glycolysis. Biochim Biophys Acta 1708:50–62
Atlante A, Bobba A, de Bari L, Fontana F, Calissano P, Marra E, Passarella S (2006) Caspase-dependent alteration of the ADP/ATP translocator triggers the mitochondrial permeability transition which is not required for the low-potassium dependent apoptosis of cerebellar granule cells. J Neurochem 97:1166–1181
Atlante A, de Bari L, Bobba A, Marra E, Passarella S (2007) Transport and metabolism of L-lactate occur in mitochondria from cerebellar granule ce lls and are modified in cells undergoing low potassium dependent apoptosis. Biochim Biophys Acta 1767:1285–1299
Atlante A, Amadoro G, Bobba A, de Bari L, Corsetti V, Pappalardo G, Marra E, Calissano P, Passarella S (2008) A peptide containing residues 26-44 of tau protein impairs mitochondrial oxidative phosphorylation acting at the level of the adenine nucleotide translocator. Biochim Biophys Acta 1777:1289–1300
Aubert A, Costalat R, Magistretti PJ, Pellerin L (2005) Brain lactate kinetics. Modelling evidence for neuronal lactate uptake upon activation. Proc Natl Acad Sci USA 102:16448–16453
Bélanger M, Yang J, Petit JM, Laroche T, Magistretti PJ, Allaman I (2011) Role of the glyoxalase system in astrocyte-mediated neuroprotection. J Neurosci 31:18338–18352
Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, Lee JM, Holtzman DM (2011) Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nat Neurosci 14:750–756
Bigl M, Bleyl AD, Zedlick D, Arendt T, Bigl V, Eschrich K (1996) Changes of activity and isoenzyme pattern of phosphofructokinase in the brains of patients with Alzheimer’s disease. J Neurochem 67:1164–1171
Bigl M, Bruckner MK, Arendt T, Bigl V, Eschrich K (1999) Activities of key glycolytic enzymes in the brains of patients with Alzheimer’s disease. J Neural Transm 106:499–511
Blass JP (2001) Brain metabolism and brain disease: is metabolic deficiency the proximate cause of Alzheimer dementia? J Neurosci Res 66:851–856
Bobba A, Atlante A, Azzariti A, Sgaramella G, Calissano P, Marra E (2004) Mitochondrial impairment induces excitotoxic death in cerebellar granule cells. Int J Mol Med 13:873–876
Bobba A, Petragallo VA, Marra E, Atlante A (2010) Alzheimer’s proteins, oxidative stress, and mitochondrial dysfunction interplay in a neuronal model of Alzheimer’s disease. Int J Alzheimers Dis. doi:10.4061/2010/621870
Bobba A, Amadoro G, Valenti D, Corsetti V, Lassandro R, Atlante A (2013) Mitochondrial respiratory chain Complexes I and IV are impaired by β-amyloid via direct interaction and through Complex I-dependent ROS production, respectively. Mitochondrion 13:298–311
Bobba A, Amadoro G, La Piana G, Calissano P, Atlante A (2015a) Glycolytic enzyme upregulation and numbness of mitochondrial activity characterize the early phase of apoptosis in cerebellar granule cells. Apoptosis 20:10–28
Bobba A, Amadoro G, La Piana G, Petragallo VA, Calissano P, Atlante A (2015b) Glucose-6-phosphate tips the balance in modulating apoptosis in cerebellar granule cells. FEBS Lett 589:651–658
Bolaños JP, Almeida A, Moncada S (2010) Glycolysis: a bioenergetic or a survival pathway? Trends Biochem Sci 35:145–149. (Review)
Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C et al (2007) A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell 11:37–51
Bouras C, Hof PR, Giannakopoulos P, Michel JP, Morrison JH (1994) Regional distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of elderly patients: a quantitative evaluation of a one-year autopsy population from a geriatric hospital. Cereb Cortex 4:138–150
Brooks GA (2009) Cell–cell and intracellular lactate shuttles. J Physiol 587:5591–5600
Brooks WM, Lynch PJ, Ingle CC, Hatton A, Emson PC, Faull RL, Starkey MP (2007) Gene expression profiles of metabolic enzyme transcripts in Alzheimer’s disease. Brain Res 1127:127–135
Cai Q, Tammineni P (2016) Mitochondrial aspects of synaptic dysfunction in Alzheimer’s disease. J Alzheimers Dis 1–17. doi:10.3233/JAD-160726
Caldwell CC, Yao J, Brinton RD (2015) Targeting the prodromal stage of Alzheimer’s disease: bioenergetic and mitochondrial opportunities. Neurotherapeutics 12:66–80
Calissano P, Matrone C, Amadoro G (2009) Apoptosis and in vitro Alzheimer disease neuronal models. Commun Integra Biol 2:163–169
Canu N, Calissano P (2003) In vitro cultured neurons for molecular studies correlating apoptosis with events related to Alzheimer disease. Cerebellum 2:270–278
Cardoso SM, Santos S, Swerdlow RH, Oliveira CR (2001) Functional mitochondria are required for amyloid beta-mediated neurotoxicity. FASEB J 15:1439–1441
Casley CS, Canevari L, Land JM, Clark JB, Sharpe MA (2002) Beta-amyloid inhibits integrated mitochondrial respiration and key enzyme activities. J Neurochem 80:91–100
Chen Z, Zhong C (2013) Decoding Alzheimer’s disease from perturbed cerebral glucose metabolism: implications for diagnostic and therapeutic strategies. Prog Neurobiol 108:21–43
Chen J, Cohen ML, Lerner AJ, Yang Y, Herrup K (2010) DNA damage and cell cycle events implicate cerebellar dentate nucleus neurons as targets of Alzheimer’s disease. Mol Neurodegener 5:60
Correia-Melo C, Birch J, Passos JF (2016a) Powering senescence: the ugly side of mitochondria. Cell Cycle 11:1–2
Correia-Melo C, Marques FD, Anderson R, Hewitt G, Hewitt R, Cole J, Carroll BM et al (2016b) Mitochondria are required for pro-ageing features of the senescent phenotype. EMBO J 35:724–742
Cunnane S, Nugent S, Roy M, Courchesne-Loyer A, Croteau E, Tremblay S, Castellano A, Pifferi F, Bocti C et al (2011) Brain fuel metabolism, aging, and Alzheimer’s disease. Nutrition 27:3–20
Demetrius LA, Driver J (2013) Alzheimer’s as a metabolic disease. Biogerontology 14:641–649
Demetrius LA, Driver JA (2015) Preventing Alzheimer’s disease by means of natural selection. J R Soc Interface 12:20140919. (Review)
Demetrius LA, Simon DK (2012) An inverse-Warburg effect and the origin of Alzheimer’s disease. Biogerontology 13:583–594
Demetrius LA, Simon DK (2013) The inverse association of cancer and Alzheimer’s: a bioenergetic mechanism. J R Soc Interface 10:20130006
Dickson DW, Wertkin A, Mattiace LA, Fier E, Kress Y, Davies P et al (1990) Ubiquitin immunoelectron microscopy of dystrophic neurites in cerebellar senile plaques of Alzheimer’s disease. Acta Neuropathol 79:486–493
D’Mello SR, Galli C, Ciotti T, Calissano P (1993) Induction of apoptosis in cerebellar granule neurons by low potassium: inhibition of death by insulin-like growth factor I and cAMP. Proc Natl Acad Sci USA 90:10989–10993
Drachman DA (2006) Aging of the brain, entropy, and Alzheimer disease. Neurology 67:1340–1352
Driver JA (2014) Inverse association between cancer and neurodegenerative disease: review of the epidemiologic and biological evidence. Biogerontology 15:547–557. (Review)
Driver JA, Beiser A, Au R, Kreger BE, Splansky GL, Kurth T, Kiel DP et al (2012) Inverse association between cancer and Alzheimer’s disease: results from the Framingham Heart Study. BMJ 344:e1442
Eckert A, Marques CA, Keil U, Schüssel K, Müller WE (2003) Increased apoptotic cell death in sporadic and genetic Alzheimer’s disease. Ann N Y Acad Sci 1010:604–609. (Review)
Eckert A, Schulz KL, Rhein V, Götz J (2010) Convergence of amyloid-beta and tau pathologies on mitochondria in vivo. Mol Neurobiol 41:107–114
Fattoretti P, Balietti M, Casoli T, Giorgetti B, Di Stefano G, Bertoni-Freddari C, Lattanzio F, Sensi SL (2010) Decreased numeric density of succinic dehydrogenase-positive mitochondria in CA1 pyramidal neurons of 3xTg-AD mice. Rejuvenation Res 13:144–147
Fuchsberger T, Martínez-Bellver S, Giraldo E, Teruel-Martí V, Lloret A, Viña J (2016) Aβ induces excitotoxicity mediated by APC/C-Cdh1 depletion that can be prevented by glutaminase inhibition promoting neuronal survival. Sci Rep 6:31158
Fujii S, Beutler E (1985) High glucose concentrations partially release hexokinase from inhibition by glucose 6-phosphate. Proc Natl Acad Sci USA 82:1552–1554
Fukutani Y, Cairns NJ, Rossor MN, Lantos PL (1996) Purkinje cell loss and astrocytosis in the cerebellum in familial and sporadic Alzheimer’s disease. Neurosci Lett 214:33–36
Gatenby RA, Gillies RJ (2004) Why do cancers have high aerobic glycolysis? Nat Rev Cancer 4:891–899. (Review)
Gibson GE, Sheu KF, Blass JP (1998) Abnormalities of mitochondrial enzymes in Alzheimer disease. J Neural Transm 105:855–870
Gogvadze V, Zhivotovsky B, Orrenius S (2010) The Warburg effect and mitochondrial stability in cancer cells. Mol Aspects Med 31:60–74
Harris RA, Tindale L, Lone A, Singh O, Macauley SL, Stanley M, Holtzman DM, Bartha R, Cumming RC (2016) Aerobic glycolysis in the frontal cortex correlates with memory performance in wild-type mice but not the APP/PS1 mouse model of cerebral amyloidosis. J Neurosci 36:1871–1878
Hashimoto T, Hussien R, Brooks GA (2006) Colocalization of MCT1, CD147, and LDH in mitochondrial inner membrane of L6 muscle cells: evidence of a mitochondrial lactate oxidation complex. Am J Physiol 290:E1237–E1244
Hashimoto T, Hussien R, Cho HS, Kaufer D, Brooks GA (2008) Evidence for the mitochondrial lactate oxidation complex in rat neurons: demonstration of an essential component of brain lactate shuttles. PLoS ONE 3:e2915
Heneka MT, Rodríguez JJ, Verkhratsky A (2010) Neuroglia in neurodegeneration. Brain Res Rev 63:189–211
Herrero-Mendez A, Almeida A, Fernández E, Maestre C, Moncada S, Bolaños JP (2009) The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat Cell Biol 11:747–752
Hsu PP, Sabatini DM (2008) Cancer cell metabolism: Warburg and beyond. Cell 134:703–707
Hu H, Tan CC, Tan L, Yu JT (2016) A mitocentric view of Alzheimer’s disease. Mol Neurobiol. doi:10.1007/s12035-016-0117-7
Ishii K, Sasaki M, Kitagaki H, Yamaji S, Sakamoto S, Matsuda K et al (1997) Reduction of cerebellar glucose metabolism in advanced Alzheimer’s disease. J Nucl Med 38:925–928
Iwangoff P, Armbruster R, Enz A, Meier-Ruge W (1980) Glycolytic enzymes from human autoptic brain cortex: normal aged and demented cases. Mech Ageing Dev 14:203–209
Jack C Jr, Knopman D, Jagust W, Shaw L, Aisen P, Weiner M, Petersen R, Trojanowski J (2010) Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 9:119–128
Janson J, Laedtke T, Parisi JE, O’Brien P, Petersen RC, Butler PC (2004) Increased risk of type 2 diabetes in Alzheimer disease. Diabetes 53:474–481
Kachel P, Trojanowicz B, Sekulla C, Prenzel H, Dralle H, Hoang-Vu C (2015) Phosphorylation of pyruvate kinase M2 and lactate dehydrogenase A by fibroblast growth factor receptor 1 in benign and malignant thyroid tissue. BMC Cancer 15:140
Kapogiannis D, Mattson MP (2011) Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurol 10:187–198
Karran E, De Strooper B (2016) The amyloid cascade hypothesis: are we poised for success or failure? J Neurochem 139(Suppl 2):237–252
Karran E, Mercken M, De Strooper B (2011) The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat Rev Drug Discov 10:698–712
Kopeikina KJ, Hyman BT, Spires-Jones TL (2012) Soluble forms of tau are toxic in Alzheimer’s disease. Transl Neurosci 3:223–233
Krako N, Magnifico MC, Arese M, Meli G, Forte E, Lecci A, Manca A, Giuffre` A, Mastronicola D, Sarti P, Cattaneo A (2013) Characterization of mitochondrial dysfunction in the 7PA2 cell model of Alzheimer’s disease. J Alzheimer’s Dis 37:747–758
Kuhla B, Boeck K, Schmidt A, Ogunlade V, Arendt T, Münch G, Lüth HJ (2007) Age- and stage-dependent glyoxalase I expression and its activity in normal and Alzheimer’s disease brains. Neurobiol Aging 28:29–41
Lemire J, Mailloux RJ, Appanna VD (2008) Mitochondrial lactate dehydrogenase is involved in oxidative-energy metabolism in human astrocytoma cells (CCF-STTG1). PLoS ONE 3:e1550
Liang WS, Reiman EM, Valla J, Dunckley T, Beach TG, Grover A, Niedzielko TL et al (2008) Alzheimer’s disease is associated with reduced expression of energy metabolism genes in posterior cingulate neurons. Proc Natl Acad Sci USA 105:4441–4446
Luque FA, Jaffe SL (2009) The molecular and cellular pathogenesis of dementia of the Alzheimer’s type an overview. Int Rev Neurobiol 84:151–165
Magistretti PJ, Allaman I (2015) A cellular perspective on brain energy metabolism and functional imaging. Neuron 86:883–901
Manczak M, Reddy PH (2012) Abnormal interaction of VDAC1 with amyloid beta and phosphorylated tau causes mitochondrial dysfunction in Alzheimer’s disease. Hum Mol Genet 21:5131–5146
Manczak M, Reddy PH (2013) RNA silencing of genes involved in Alzheimer’s disease enhances mitochondrial function and synaptic activity. Biochim Biophys Acta 1832:2368–2378
Manczak M, Park BS, Jung Y, Reddy PH (2004) Differential expression of oxidative phosphorylation genes in patients with Alzheimer’s disease: implications for early mitochondrial dysfunction and oxidative damage. Neuromol Med 5:147–162
Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH (2006) Mitochondria are a direct site of A beta accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet 15:1437–1449
Maragakis NJ, Rothstein JD (2006) Mechanisms of disease: astrocytes in neurodegenerative disease. Nat Clin Pract Neurol 2:679–689
Marcus DL, Freedman ML (1997) Decreased brain glucose metabolism in microvessels from patients with Alzheimer’s disease. Ann NY Acad Sci 826:248–253
Marcus DL, de Leon MJ, Goldman J, Logan J, Christman DR, Wolf AP, Fowler JS, Hunter K, Tsai J, Pearson J (1989) Altered glucose metabolism in microvessels from patients with Alzheimer’s disease. Ann Neurol 26:91–94
Mattiace LA, Davies P, Yen SH, Dickson DW (1990) Microglia in cerebellar plaques in Alzheimer’s disease. Acta Neuropathol 80:493–498
McFate T, Mohyeldin A, Lu H, Thakar J, Henriques J, Halim ND, Wu H, Schell MJ, Tsang TM, Teahan O, Zhou S et al (2008) Pyruvate dehydrogenase complex activity controls metabolic and malignant phenotype in cancer cells. J Biol Chem 283:22700–22708
Morais VA, De Strooper B (2010) Mitochondria dysfunction and neurodegenerative disorders: cause or consequence. J Alzheimers Dis 20(Suppl. 2):S255–263
Moreira PI, Carvalho C, Zhu X, Smith MA, Perry G (2010a) Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim Biophys Acta 1802:2–10
Moreira PI, Zhu X, Wang X, Lee HG, Nunomura A, Petersen RB, Perry G, Smith MA (2010b) Mitochondria: a therapeutic target in neurodegeneration. Biochim Biophys Acta 1802:212–220
Mortilla M, Sorbi S (1990) Hexokinases in Alzheimer’s disease. Medizina (Firenze) 10:168–169
Nehlig A, Coles JA (2007) Cellular pathways of energy metabolism in the brain: is glucose used by neurons or astrocytes? Glia 55:1238–1250
Newington JT, Pitts A, Chien A, Arseneault R, Schubert D, Cumming RC (2011) Amyloid beta resistance in nerve cell lines is mediated by the warburg effect. PLoS ONE 6:e19191
Newington JT, Rappon T, Albers S, Wong DY, Rylett RJ, Cumming RC (2012) Overexpression of pyruvate dehydrogenase kinase 1 and lactate dehydrogenase A in nerve cells confers resistance to amyloid beta and other toxins by decreasing mitochondrial respiration and ROS production. J Biol Chem 287:37245–37258
Newington JT, Harris RA, Cumming RC (2013) Reevaluating metabolism in Alzheimer’s disease from the perspective of the astrocyte-neuron lactate shuttle model. J Neurodeg Dis 2013:234572
Newman LA, Korol DL, Gold PE (2011) Lactate produced by glycogenolysis in astrocytes regulates memory processing. PLoS ONE 6:e28427
Nicholls DG (2002) Mitochondrial function and dysfunction in the cell: its relevance to aging and aging-related disease. Int J Biochem Cell Biol 34:1372–1381. (Review)
O’brien J, Kla KM, Hopkins IB, Malecki EA, McKenna MC (2007) Kinetic parameters and lactate dehydrogenase isozyme activities support possible lactate utilization by neurons. Neurochem Res 32:597–607
Ossenkoppele R, van der Flier WM, Verfaillie SC, Vrenken H, Versteeg A, van Schijndel RA et al (2014) Long-term effects of amyloid, hypometabolism, and atrophy on neuropsychological functions. Neurology 82:1768–1775
Pagani L, Eckert A (2011) Amyloid-beta interaction with mitochondria. Int J Alzheimers Dis 2011:925050
Passarella S, de Bari L, Valenti D, Pizzuto R, Paventi G, Atlante A (2008) Mitochondria and l-lactate metabolism. FEBS Lett 582:3569–3576
Pedros I, Patraca I, Martinez N, Petrov D, Sureda FX, Auladell C, Beas-Zarate C, Folch J (2016) Molecular links between early energy metabolism alterations and Alzheimer’s disease. Front Biosci (Landmark Ed) 21:8–19. (Review)
Pellerin L, Magistretti PJ (1994) Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA 91:10625–10629
Pettegrew JW, Panchalingam K, Klunk WE, McClure RJ, Muenz LR (1994) Alterations of cerebral metabolism in probable Alzheimer’s disease: a preliminary study. Neurobiol Aging 15:117–132
Poisnel G, Hérard AS, El Tannir El Tayara N, Bourrin E, Volk A, Kober F, Delatour B, Delzescaux T, Debeir T et al (2012) Increased regional cerebral glucose uptake in an APP/PS1 model of Alzheimer’s disease. Neurobiol Aging 33:1995–2005
Price JL, Morris JC (1999) Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol 45:358–368
Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP (2013) The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement 9:63.e–75.e
Proia P, Di Liegro CM, Schiera G, Fricano A, Di Liegro I (2016) Lactate as a metabolite and a regulator in the central nervous system. Int J Mol Sci. doi:10.3390/ijms17091450
Rao VS, van Duijn CM, Connor-Lacke L, Cupples LA, Growdon JH, Farrer LA (1994) Multiple etiologies for Alzheimer disease are revealed by segregation analysis. Am J Hum Genet 55:991–1000
Reddy PH (2005) Amyloid precursor protein-mediated free radicals and oxidative damage: implications for the development and progression of Alzheimer’s disease. J Neurochem 96:1–13. (Review)
Reddy PH (2013) Is the mitochondrial outer membrane protein VDAC1 therapeutic target for Alzheimer’s disease? Biochim Biophys Acta 1832:67–75
Redjems-Bennani N, Jeandel C, Lefebvre E, Blain H, Vidailhet M, Guéant JL (1998) Abnormal substrate levels that depend upon mitochondrial function in cerebrospinal fluid from Alzheimer patients. Gerontology 44:300–304
Rodriguez-Rodriguez P, Fernandez E, Almeida A, Bolaños JP (2012) Excitotoxic stimulus stabilizes PFKFB3 causing pentose-phosphate pathway to glycolysis switch and neurodegeneration. Cell Death Differ 19:1582–1589
Rohn TT, Head E (2008) Caspase activation in Alzheimer’s disease: early to rise and late to bed. Rev Neurosci 19:383–393
Rossi D, Volterra A (2009) Astrocytic dysfunction: insights on the role in neurodegeneration. Brain Res Bull 80:224–232
Santos RX, Correia SC, Wang X, Perry G, Smith MA, Moreira PI, Zhu X (2010) Alzheimer's disease: diverse aspects of mitochondrial malfunctioning. Int J Clin Exp Pathol 3:570–581
Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, Van der Flier WM (2016) Alzheimer’s disease. Lancet 388:505–517
Schubert D (2005) Glucose metabolism and Alzheimer’s disease. Ageing Res Rev 4:240–257
Schurr A (2006) Lactate: the ultimate cerebral oxidative energy substrate. J Cerebr Blood F Met 26:142–152
Schwab MA, Kölker S, van den Heuvel LP, Sauer S, Wolf NI, Rating D, Hoffmann GF, Smeitink JA, Okun JG (2005) Optimized spectrophotometric assay for the completely activated pyruvate dehydrogenase complex in fibroblasts. Clin Chem 51:151–160
Selkoe DJ, Hardy J (2016) The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8:595–608. (Review)
Seo AY, Joseph AM, Dutta D, Hwang JC, Aris JP, Leeuwenburgh C (2010) New insights into the role of mitochondria in aging: mitochondrial dynamics and more. J Cell Sci 123:533–542
Sheu KFR, Kim YT, Blass JP, Weksler ME (1985) An immunochemical study of the pyruvate dehydrogenase deficit in Alzheimer’s disease brain. Ann Neurol 17:444–449
Sims-Robinson C, Kim B, Rosko A, Feldman EL (2010) How does diabetes accelerate Alzheimer disease pathology? Nat Rev Neurol 6:551–559
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119:7–35
Sorbi S, Bird ED, Blass JP (1983) Decreased pyruvate dehydrogenase complex activity in Huntington and Alzheimer brain. Ann Neurol 13:72–78
Sorbi S, Mortilla M, Piacentini S, Tonini S, Amaducci L (1990) Altered hexokinase activity in skin cultured fibroblasts and leukocytes from Alzheimer’s disease patients. Neurosci Lett 117:165–168
Soucek T, Cumming R, Dargusch R, Maher P, Schubert D (2003) The regulation of glucose metabolism by HIF-1 mediates a neuroprotective response to amyloid β peptide. Neuron 39:43–56
Sun L, Shukair S, Naik TJ, Moazed F, Ardehali H (2008) Glucose phosphorylation and mitochondrial binding are required for the protective effects of hexokinases I and II. Mol Cell Biol 28:1007–1017
Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM (2011) Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144:810–823
Swerdlow RH, Burns JM, Khan SM (2010) The Alzheimer’s disease mitochondrial cascade hypothesis. J Alzh Dis 20(Suppl 2):S265–S279
Vaishnavi SN, Vlassenko AG, Rundle MM, Snyder AZ, Mintun MA, Raichle ME (2010) Regional aerobic glycolysis in the human brain. Proc Natl Acad Sci USA 107:17757–17762
Verkhratsky A, Sofroniew MV, Messing A, deLanerolle NC, Rempe D, Rodríguez JJ, Nedergaard M (2012) Neurological diseases as primary gliopathies: a reassessment of neurocentrism. ASN Neuro 4:e00082
Vlassenko AG, Raichle ME (2015) Brain aerobic glycolysis functions and Alzheimer’s disease. Clin Transl Imaging 3:27–37
Vlassenko AG, Vaishnavi SN, Couture L, Sacco D, Shannon BJ, Mach RH, Morris JC, Raichle ME, Mintun MA (2010) Spatial correlation between brain aerobic glycolysis and amyloid-beta (Abeta) deposition. Proc Natl Acad Sci USA 107:17763–17767
Warburg O (1956) On respiratory impairment in cancer cells. Science 124:269–270
Wegiel J, Wisniewski HM, Dziewiatkowski J, Badmajew E, Tarnawski M, Reisberg B et al (1999) Cerebellar atrophy in Alzheimer’s disease-clinicopathological correlations. Brain Res 818:41–50
Xie J, Wang BS, Yu DH, Lu Q, Ma J, Qi H, Fang C, Chen HZ (2011) Dichloroacetate shifts the metabolism from glycolysis to glucose oxidation and exhibits synergistic growth inhibition with cisplatin in HeLa cells. Int J Oncol 38:409–417
Xu RH, Pelicano H, Zhou Y, Carew JS, Feng L, Bhalla KN, Keating MJ, Huang P (2005) Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res 65:613–621
Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD (2009) Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA 106:14670–14675
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Atlante, A., de Bari, L., Bobba, A. et al. A disease with a sweet tooth: exploring the Warburg effect in Alzheimer’s disease. Biogerontology 18, 301–319 (2017). https://doi.org/10.1007/s10522-017-9692-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-017-9692-x