Abstract
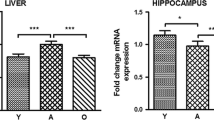
Dietary restriction (DR) exerts significant beneficial effects in terms of aging and age-related diseases in many organisms including humans. The present study aimed to examine the influence of long-term DR on the BDNF system at the transcriptional and translational levels in the cortex and hippocampus of middle-aged (12-month-old) and aged (24-month-old) male Wistar rats. The obtained results revealed that the DR upregulated the expression of exon-specific BDNF transcripts in both regions, followed by elevated levels of mBDNF only in the cortex in middle-aged animals. In aged animals, DR modulated BDNF protein levels by increasing proBDNF and by declining mBDNF levels. Additionally, elevated levels of the full-length TrkB accompanied by a decreased level of the less-glycosylated TrkB protein were observed in middle-aged rats following DR, while in aged rats, DR amplified only the expression of the less-glycosylated form of TrkB. The levels of phosphorylated TrkBY816 were stable during aging regardless of feeding. Reduced levels of p75NTR were detected in both regions of middle-aged DR-fed animals, while a significant increase was measured in the cortex of aged DR-fed rats. These findings shed additional light on DR as a modulator of BDNF system revealing its disparate effects in middle-aged and aged animals. Given the importance of the proBDNF/BDNF circuit-level expression in different brain functions and various aspects of behavior, it is necessary to further elucidate the optimal duration of the applied dietary regimen with regard to the animal age in order to achieve its most favorable effects.





Similar content being viewed by others
References
Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T (2007) Mouse and rat BDNF gene structure and expression revisted. J Neurosci Res 85:525–535
Andrade JP, Mesquita R, Assunção M, Pereira PA (2006) Effects of food restriction on synthesis and expression of brain-derived neurotrophic factor and tyrosine kinase B in dentate gyrus granule cells of adult rats. Neurosci Lett 399:135–140
Asha Devi S (2009) Aging brain: prevention of oxidative stress by vitamin E and exercise. Sci World J 9:366–372
Baj G, Leone E, Chao MV, Tongiorgi E (2011) Spatial segregation of BDNF transcripts enables BDNF to differentially shape distinct dendritic compartments. Proc Natl Acad Sci USA 108:16813–16818
Baker SJ, Reddy EP (1996) Transducers of life and death: TNF receptor superfamily and associated proteins. Oncogene 12:1–9
Barker PA (2007) High affinity not in the vicinity? Neuron 53:1–4
Barker PA (2009) Whither proBDNF? Nat Neurosci 12:105–106
Barnes P, Thomas KL (2008) Proteolysis of proBDNF is a key regulator in the formation of memory. PLoS One 3:e3248
Beauchene RE, Bales CW, Bragg CS (1986) Effect of age of initiation of feed restriction on growth, body composition, and longevity of rats. J Gerontol 41:13–19
Brito V, Puigdellívol M, Giralt A, del Toro D, Alberch J, Ginés S (2013) Imbalance of p75(NTR)/TrkB protein expression in Huntington’s disease: implication for neuroprotective therapies. Cell Death Dis 4:e595
Calabrese F, Guidotti G, Racagni G, Riva MA (2013) Reduced neuroplasticity in aged rats: a role for the neurotrophin brain-derived neurotrophic factor. Neurobiol Aging 34:2768–2776
Duan W, Lee J, Guo Z, Mattson MP (2001) Dietary restriction stimulates BDNF production in the brain and thereby protects neurons against excitotoxic injury. J Mol Neurosci 16:1–12
Duan W, Guo Z, Jiang H, Ware M, Mattson MP (2003) Reversal of behavioral and metabolic abnormalities, and insulin resistance syndrome, by dietary restriction in mice deficient in brain-derived neurotrophic factor. Endocrinology 144:2446–2453
Fontán-Lozano A, López-Lluch G, Delgado-García JM, Navas P, Carrión AM (2008) Molecular bases of caloric restriction regulation of neuronal synaptic plasticity. Mol Neurobiol 38:167–177
Fryer RH, Kaplan DR, Feinstein SC, Radeke MJ, Grayson DR, Kromer LF (1996) Developmental and mature expression of full-length and truncated TrkB receptors in the rat forebrain. J Comp Neurol 374:21–40
Fusco S, Pani G (2013) Brain response to calorie restriction. Cell Mol Life Sci 70:3157–3170
Fusco S, Ripoli C, Podda MV, Ranieri SC, Leone L, Toietta G, McBurney MW, Schutz G, Riccio A, Grassi C, Galeotti T, Pani G (2012) A role for neuronal cAMP responsive-element binding (CREB)-1 in brain responses to calorie restriction. Proc Natl Acad Sci USA 109:621–626
Gelegen C, van den Heuvel J, Collier DA, Campbell IC, Oppelaar H, Hessel E, Kas MJ (2008) Dopaminergic and brain-derived neurotrophic factor signalling in inbred mice exposed to a restricted feeding schedule. Genes Brain Behav 7:552–559
Hansalik M, Skalicky M, Viidik A (2006) Impairment of water maze behaviour with ageing is counteracted by maze learning earlier in life but not by physical exercise, food restriction or housing conditions. Exp Gerontol 41:169–174
Hattiangady B, Rao MS, Shetty GA, Shetty AK (2005) Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp Neurol 195:353–371
Hof PR, Morrison JH (2004) The aging brain: morphomolecular senescence of cortical circuits. Trends Neurosci 27:607–613
Huang EJ, Reichardt LF (2003) Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem 72:609–642
Ishikawa Y, Ikeuchi T, Hatanaka H (2000) Brain-derived neurotrophic factor accelerates nitric oxide donor-induced apoptosis of cultured cortical neurons. J Neurochem 75:494–502
Jessberger S, Gage FH (2008) Stem-cell-associated structural and functional plasticity in the aging hippocampus. Psychol Aging 23:684–691
Kim HJ, Hwang JJ, Behrens MM, Snider BJ, Choi DW, Koh JY (2003) TrkB mediates BDNF-induced potentiation of neuronal necrosis in cortical culture. Neurobiol Dis 14:110–119
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lee J, Duan W, Long JM, Ingram DK, Mattson MP (2000) Dietary restriction increases the number of newly generated neural cells, and induces BDNF expression, in the dentate gyrus of rats. J Mol Neurosci 15:99–108
Lee J, Duan W, Mattson MP (2002a) Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem 82:1367–1375
Lee J, Seroogy KB, Mattson MP (2002b) Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J Neurochem 80:539–547
Markowska AL (1999) Life-long diet restriction failed to retard cognitive aging in Fischer-344 rats. Neurobiol Aging 20:177–189
Martin B, Pearson M, Kebejian L, Golden E, Keselman A, Bender M, Carlson O, Egan J, Ladenheim B, Cadet JL, Becker KG, Wood W, Duffy K, Vinayakumar P, Maudsley S, Mattson MP (2007) Sex-dependent metabolic, neuroendocrine, and cognitive responses to dietary energy restriction and excess. Endocrinology 148:4318–4333
Martin-Zanca D, Oskam R, Mitra G, Copeland T, Barbacid M (1989) Molecular and biochemical characterization of the human trk proto-oncogene. Mol Cell Biol 9:24–33
Matsuzaki J, Kuwamura M, Yamaji R, Inui H, Nakano Y (2001) Inflammatory responses to lipopolysaccharide are suppressed in 40 % energy-restricted mice. J Nutr 131:2139–2144
Mladenovic Djordjevic A, Perovic M, Tesic V, Tanic N, Rakic L, Ruzdijic S, Kanazir S (2010) Long-term dietary restriction modulates the level of presynaptic proteins in the cortex and hippocampus of the aging rat. Neurochem Int 56:250–255
Mladenovic A, Perovic M, Tanic N, Petanceska S, Ruzdijic S, Kanazir S (2007) Dietary restriction modulates alpha-synuclein expression in the aging rat cortex and hippocampus. Synapse 61:790–794
Morioka N, Yoshida Y, Nakamura Y, Hidaka N, Hisaoka-Nakashima K, Nakata Y (2013) The regulation of exon-specific brain-derived neurotrophic factor mRNA expression by protein kinase C in rat cultured dorsal root ganglion neurons. Brain Res 1509:20–31
Nagappan G, Zaitsev E, Senatorov VV Jr, Yang J, Hempstead BL, Lu B (2009) Control of extracellular cleavage of ProBDNF by high frequency neuronal activity. Proc Natl Acad Sci USA 106:1267–1272
Newton IG, Forbes ME, Legault C, Johnson JE, Brunso-Bechtold JK, Riddle DR (2005) Caloric restriction does not reverse aging-related changes in hippocampal BDNF. Neurobiol Aging 26:683–688
Noble EE, Billington CJ, Kotz CM, Wang C (2011) The lighter side of BDNF. Am J Physiol Regul Integr Comp Physiol 300:1053–1069
Okada M, Nakanishi H, Amamoto T, Urae R, Ando S, Yazawa K, Fujiwara M (2003) How does prolonged caloric restriction ameliorate age-related impairment of long-term potentiation in the hippocampus? Brain Res Mol Brain Res 111:175–181
Pang PT, Lu B (2004) Regulation of late-phase LTP and long-term memory in normal and aging hippocampus: role of secreted proteins tPA and BDNF. Ageing Res Rev 3:407–430
Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL, Lu B (2004) Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science 306:487–491
Park SK, Prolla TA (2005) Lessons learned from gene expression profile of aging and caloric restriction. Aging Res Rev 4:55–65
Pattabiraman PP, Tropea D, Chiaruttini C, Tongiorgi E, Cattaneo A, Domenici L (2005) Neuronal activity regulates the developmental expression and subcellular localization of cortical BDNF mRNA isoforms in vivo. Mol Cell Neurosci 28:556–570
Perovic M, Tesic V, Djordjevic AM, Smiljanic K, Loncarevic-Vasiljkovic N, Ruzdijic S, Kanazir S (2013) BDNF transcripts, proBDNF and proNGF, in the cortex and hippocampus throughout the life span of the rat. Age (Dordr). 35:2057–2070
Smiljanic K, Vanmierlo T, Djordjevic AM, Perovic M, Ivkovic S, Lütjohann D, Kanazir S (2014) Cholesterol metabolism changes under long-term dietary restrictions while the cholesterol homeostasis remains unaffected in the cortex and hippocampus of aging rats. Age (Dordr) 36:9654
Speakman JR, Hambly C (2007) Starving for life: what animal studies can and cannot tell us about the use of caloric restriction to prolong human lifespan. J Nutr 137:1078–1086
Tanic N, Perovic M, Mladenovic A, Ruzdijic S, Kanazir S (2007) Effects of aging, dietary restriction and glucocorticoid treatment on housekeeping gene expression in rat cortex and hippocampus-evaluation by real time RT-PCR. J Mol Neurosci 32:38–46
Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME (1998) Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron 20:709–726
Tapia-Arancibia L, Aliaga E, Silhol M, Arancibia S (2008) New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Res Rev 59:201–220
Teng HK, Teng KK, Lee R, Wright S, Teva S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS, Kraemer RT, Nykjaer A, Hempstead BL (2005) Pro-BDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci 25:5455–5463
Unsain N, Nunez N, Anastasia A, Masco DH (2008) Status epilepticus induces a TrkB to p75 neurotrophin receptor switch and increases brain-derived neurotrophic factor interaction with p75 neurotrophin receptor: an initial event in neuronal injury induction. Neuroscience 154:978–993
Watson FL, Porcionatto MA, Bhattacharyya A, Stiles CD, Segal RA (1999) TrkA glycosylation regulates receptor localization and activity. J Neurobiol 39:323–336
Yanai S, Okaichi Y, Okaichi H (2004) Long-term dietary restriction causes negative effects on cognitive functions in rats. Neurobiol Aging 25:325–332
Yilmazer-Hanke DM (2008) Morphological correlates of emotional and cognitive behaviour: insights from studies on inbred and outbred rodent strains and their crosses. Behav Pharmacol 19:403–434
Acknowledgments
This work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant No. ON173056) and NIH Grant R03AG046216. We would like to thank Dr. Sanja Ivkovic for the critical reading and helpful comments regarding this paper.
Conflict of interest
The authors have no conflicts of interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Kosara Smiljanic and Vesna Pesic have equally contribution to this work.
Rights and permissions
About this article
Cite this article
Smiljanic, K., Pesic, V., Mladenovic Djordjevic, A. et al. Long-term dietary restriction differentially affects the expression of BDNF and its receptors in the cortex and hippocampus of middle-aged and aged male rats. Biogerontology 16, 71–83 (2015). https://doi.org/10.1007/s10522-014-9537-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-014-9537-9