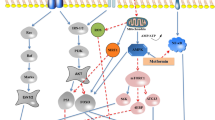
Abstract
FoxO activity and modifications, such as its phosphorylation, acetylation, and methylation, may help drive the expression of genes involved in combating oxidative stress by causing the epigenetic modifications, and thus, preserve cellular function during aging and age-related diseases, such as diabetes, cancer, and Alzheimer disease. Insulin signaling has been postulated to influence the aging process by increasing resistance to oxidative stress, and slowing the accumulation of oxidative damage. Some antioxidative effects are mediated by a conserved family of forkhead box transcription factors (FoxOs), which in the absence of insulin signaling freely bind to promoters of antioxidant enzymes, superoxide dismutase, and catalase. On the other hand, calorie restriction (CR) extends the lifespans of several species via the insulin pathway, and extends longevity and healthspan in diverse species via a conserved mechanism. CR enhances adaptive stress responses at the cellular and organism levels and extends lifespan in a FoxO-independent manner. Thus, increased modification of FoxO is modulated via the hyperinsulinemia-induced PI3K/Akt pathway during aging, and CR reverses this process. Accordingly, FoxO plays an important role in maintenance of metabolic homeostasis and removal of oxidative stress in the aging process and in the effect of CR on lifespan.

Similar content being viewed by others
References
Accili D, Arden KC (2004) FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117:421–426
Almeida M, Han L, Martin-Millan M, O’Brien CA, Manolagas SC (2007) Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting beta-catenin from T cell factor-to forkhead box O-mediated transcription. J Biol Chem 282:27298–27305
Aoki M, Jiang H, Vogt PK (2004) Proteasomal degradation of the FoxO1 transcriptional regulator in cells transformed by the P3k and Akt oncoproteins. Proc Natl Acad Sci USA 101:13613–13617
Asada S, Daitoku H, Matsuzaki H, Saito T, Sudo T, Mukai H, Iwashita S, Kako K, Kishi T, Kasuya Y, Fukamizu A (2007) Mitogen-activated protein kinases, Erk and p38, phosphorylate and regulate Foxo1. Cell Signal 19:519–527
Bakker WJ, Blazquez-Domingo M, Kolbus A, Besooyen J, Steinlein P, Beug H, Coffer PJ, Lowenberq B, von Lindern M, van Dijk TB (2004) FoxO3a regulates erythroid differentiation and induces BTG1, an activator of protein arginine methyl transferase I. J Cell Biol 164:175–184
Balaban R, Nemoto S, Finkel T (2005) Mitochondria, oxidants, and aging. Cell 120:483–495
Barthel A, Schmoll D, Kruger KD, Roth RA, Joost HG (2002) Regulation of the forkhead transcription factor FKHR (FOXO1a) by glucose starvation and AICAR, an activator of AMP-activated protein kinase. Endocrinology 143:3183–3186
Barthel A, Schmoll D, Unterman TG (2005) FoxO proteins in insulin action and metabolism. Trends Endocrinol Metab 16:183–189
Barthelemy C, Henderson CE, Pettmann B (2004) Foxo3a induces motoneuron death through the Fas pathway in cooperation with JNK. BMC Neurosci 5:48
Berg BN, Simms HS (1960) Nutrition and longevity in the rat. II. Longevity and onset of disease with different levels of food intake. J Nutr 71:255–263
Biggs WH, Meisenhelder J, Hunter T, Cavenee WK, Arden KC (1999) Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci USA 96:7421–7426
Bordone L, Guarente L (2005) Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol 6:298–305
Brenkman AB, de Keizer PL, van den Broek NJ, Jochemsen AG, Burgering BM (2008) Mdm2 induces mono-ubiquitination of FOXO4. PLoS One 3:e2819
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME (1999) Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell 96:857–868
Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME (2004) Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303:2011–2015
Calabrese V, Cornelius C, Cuzzocrea S, Iavicoli I, Rizzarelli E, Calabrese EJ (2011) Hormesis, cellular stress response and vitagenes as critical determinants in aging and longevity. Mol Aspects Med 32:279–304
Calvanese V, Lara E, Kahn A, Fraga MF (2009) The role of epigenetics in aging and age-related diseases. Ageing Res Rev 8:268–276
Cameron AR, Anton S, Melville L, Houston NP, Dayal S, McDougall GJ, Stewart D, Rena G (2008) Black tea polyphenols mimic insulin/insulin-like growth factor-1 signaling to the longevity factor FoxO1a. Aging Cell 7:69–77
Campisi J, di Fagagna FD (2007) Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 8:729–740
Canto’ C, Auwerx J (2009) Caloric restriction, SIRT1 and longevity. Trends Endocrinol Metab 20:325–331
Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho PA (2003) Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science 301:215–218
Chiba T, Tsuchiya T, Komatsu T, Mori R, Hayashi H, Shimokawa I (2010) Development of calorie restriction mimetics as therapeutics for obesity, diabetes, inflammatory and neurodegenerative diseases. Curr Genomics 11:562–567
Choi KM, Lee HL, Kwon YY, Kang MS, Lee SK, Lee CK (2013) Enhancement of mitochondrial function correlates with the extension of lifespan by caloric restriction and caloric restriction mimetics in yeast. Biochem Biophys Res Commun 441:236–242
Chung HY, Lee EK, Choi YJ, Kim JM, Kim DH, Zou Y, Kim CH, Lee J, Kim HS, Kim ND, Jung JH, Yu BP (2011) Molecular inflammation as an underlying mechanism of the aging process and age-related diseases. J Dent Res 90:830–840
Clavel S, Coldefy AS, Kurkdjian E, Salles J, Margaritis I, Derijard B (2006) Atrophy-related ubiquitin ligases, atrogin-1, and MuRF1 are up-regulated in aged rat tibialis anterior muscle. Mech Aging Dev 127:794–801
Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A (2006) Opposing activities protect against age-onset proteotoxicity. Science 313:1604–1610
Collado M, Blasco MA, Serrano M (2007) Cellular senescence in cancer and aging. Cell 130:223–233
Daitoku H, Yamagata K, Matsuzaki H, Hatta M, Fukamizu A (2003) Regulation of PGC-1 promoter activity by protein kinase B and the forkhead transcription factor FKHR. Diabetes 52:642–649
Daitoku H, Hatta M, Matsuzaki H, Aratani S, Ohshima T, Miyaqishi M, Nakajima T, Fukamizu A (2004) Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc Natl Acad Sci USA 101:10042–10047
del Peso L, Gonzalez VM, Hernandez R, Barr FG, Nunez G (1999) Regulation of the forkhead transcription factor FKHR, but not the PAX3-FKHR fusion protein, by the serine/threonine kinase Akt. Oncogene 18:7328–7333
Delpuech O, Griffiths B, East P, Essafi A, Lam EW, Burgering B, Downward J, Schulze A (2007) Induction of Mxi1-SR(alpha) by FOXO3a contributes to repression of Myc-dependent gene expression. Mol Cell Biol 27:4917–4930
Demetrius L (2005) Of mice and men. When it comes to studying ageing and the means to slow it down, mice are not just small humans. EMBO Rep 6:39–44
Demetrius L (2006) Aging in mouse and human systems: a comparative study. Ann NY Acad Sci 1067:66–82
Dixit M, Bess E, Fisslthaler B, Hartel FV, Noll T, Busse R, Fleminq I (2007) Shear stress-induced activation of the AMP-activated protein kinase regulates FoxO1a and angiopoietin-2 in endothelial cells. Cardiovasc Res 77:160–168
Dowell P, Otto TC, Adi S, Lane MD (2003) Convergence of peroxisome proliferator-activated receptor gamma and Foxo1 signaling pathways. J Biol Chem 278:45485–45491
Edstrom E, Altun M, Hagglund M, Ulfhake B (2006) Atrogin-1/MAFbx and MuRF1 are downregulated in aging-related loss of skeletal muscle. J Gerontol A Biol Sci Med Sci 61:663–674
Essers MA, Weijzen S, deVries-Smits AM, Saarloos I, de Ruiter ND, Bos JL, Burgering BM (2004) FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J 23:4802–4812
Essers MA, de Vries-Smits LM, Barker N, Polderman PE, Burgering BM, Korswagen HC (2005) Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science 308:1181–1184
Evans-Anderson HJ, Alfieri CM, Yutzey KE (2008) Regulation of cardiomyocyte proliferation and myocardial growth during development by FOXO transcription factors. Circ Res 102:686–694
Fischle W, Wang Y, Allis CD (2003) Histone and chromatin cross-talk. Curr Opin Cell Biol 15:172–183
Fontana L, Partridge L, Longo VD (2010) Extending healthy life span-from yeast to humans. Science 328:321–326
Fraga ME, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, Heine-Suñer D, Cigudosa JC, Urioste M, Benitez J, Boix-Chornet M, Sanchez-Aguilera A, Ling C, Carlsson E, Poulsen P, Vaag A, Stephan Z, Spector TD, Wu YZ, Plass C, Esteller M (2005) Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci USA 102:10604–10609
Frescas D, Valenti L, Accili D (2005) Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J Biol Chem 280:20589–20595
Fu W, Ma Q, Chen L, Li P, Zhanq M, Ramamoorthy S, Nawaz Z, Shimojima T, Wanq H, Yanq Y, Shen Z, Zhanq Y, Zhanq X, Nicosia SV, Zhanq Y, Pledqer JW, Chen J, Bai W (2009) MDM2 acts downstream of p53 as an E3 ligase to promote FOXO ubiquitination and degradation. J Biol Chem 284:13987–14000
Fukuoka M, Daitoku H, Hatta M, Matsuzaki H, Umemura S, Fukamizu A (2003) Negative regulation of forkhead transcription factor AFX (Foxo4) by CBP-induced acetylation. Int J Mol Med 12:503–508
Furukawa-Hibi Y, Yoshida-Araki K, Ohta T, Ikeda K, Motoyama N (2002) FOXO forkhead transcription factors induce G(2)-M checkpoint in response to oxidative stress. J Biol Chem 277:26729–26732
Furukawa-Hibi Y, Kobayashi Y, Chen C, Motoyama N (2005) FOXO transcription factors in cell-cycle regulation and the response to oxidative stress. Antioxid Redox Signal 7:752–760
Furuyama T, Nakazawa T, Nakano I, Mori N (2000) Identification of the differential distribution patterns of mRNAs and consensus binding sequences for mouse DAF-16 homologues. Biochem J 349:629–634
Furuyama T, Yamashita H, Kitayama K, Higami Y, Shimokawa I, Mori N (2002) Effects of aging and caloric restriction on the gene expression of Foxo1, 3, and 4 (FKHR, FKHRL1, and AFX) in the rat skeletal muscles. Microsc Res Tech 59:331–334
Galgani JE, Uauy RD, Aguirre CA, Diaz EO (2008) Effect of the dietary fat quality on insulin sensitivity. Br J Nutr 100:471–479
Giannakou ME, Partridge L (2004) The interaction between FOXO and SIRT1: tipping the balance towards survival. Trends Cell Biol 14:408–412
Giannakou ME, Goss M, Partridge L (2008) Role of dFOXO in lifespan extension by dietary restriction in Drosophila melanogaster: not required, but its activity modulates the response. Aging Cell 7:187–198
Goldberg RB (2009) Cytokine and cytokine-like inflammation markers, endothelial dysfunction, and imbalanced coagulation in development of diabetes and its complications. J Clin Endocrinol Metab 94:3171–3182
Gomis RR, Alarcon C, He W, Wang Q, Seoane J, Lash A, Massague J (2006) A FoxO-Smad synexpression group in human keratinocytes. Proc Natl Acad Sci USA 103:12747–12752
Greer EL, Oskoui PR, Banko MR, Maniar JM, Gvgi MP, Gvgi SP, Brunet A (2007a) The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem 282:30107–30119
Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, Gvgi SP, Brunet A (2007b) An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol 17:1646–1656
Guarente L, Picard F (2005) Calorie restriction–the SIR2 connection. Cell 120:473–482
Guo S, Rena G, Cichy S, He X, Cohen P, Unterman T (1999) Phosphorylation of serine 256 by protein kinase B disrupts transactivation by FKHR and mediates effects of insulin on IGF binding protein-1 promoter activity through a conserved insulin response sequence. J Biol Chem 274:17184–17192
Hardie DG (2009) AMPK: a key regulator of energy balance in the single cell and the whole organism. Int J Obes (Lond) 32:S7–S12
Hayashi H, Yamaza H, Komatsu T, Park S, Chiba T, Higami Y, Nagayasu T, Shimokawa I (2008) Calorie restriction minimizes activation of insulin signaling in response to glucose: potential involvement of the growth hormone-insulin-like growth factor 1 axis. Exp Gerontol 43:827–832
He XY, Zhao XL, Gu Q, Shen JP, Hu Y, Hu RM (2012) Calorie restriction from a young age preserves the functions of pancreatic β cells in aging rats. Tohoku J Exp Med 227:245–252
Hedrick SM (2009) The cunning little vixen: FoxO and the cycle of life and death. Nat Immunol 10:1057–1063
Hoekman MF, Jacobs FM, Smidt MP, Burbach JP (2006) Spatial and temporal expression of FoxO transcription factors in the developing and adult murine brain. Gene Expr Patterns 6:134–140
Hsu AL, Murphy CT, Kenyon C (2003) Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science 300:1142–1145
Huang H, Reqan KM, Wanq F, Wanq D, Smith DI, van Deursen JM, Tindall DJ (2005) Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proc Natl Acad Sci USA 102:1649–1654
Huang H, Regan KM, Lou Z, Chen J, Tindall DJ (2006) CDK2-dependent phosphorylation of FOXO1 as an apoptotic response to DNA damage. Science 314:294–297
Jacobs FM, van der Heide LP, Wijchers PJ, Burbach JP, Hoekman MF, Smidt MP (2003) FoxO6, a novel member of the FoxO class of transcription factors with distinct shuttling dynamics. J Biol Chem 278:35959–35967
Jian B, Yang S, Chen D, Chaudry I, Raju R (2011) Influence of aging and hemorrhage injury on Sirt1 expression: possible role of myc-Sirt1 regulation in mitochondrial function. Biochim Biophys Acta 12:1446–1451
Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB (2005) AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell 18:283–293
Kato S, Ding J, Pisck E, Jhala US, Du K (2008) COP1 functions as a FoxO1 ubiquitin E3 ligase to regulate FoxO1-mediated gene expression. J Biol Chem 283:35464–35473
Kim DH, Kim JY, Yu BP, Chung HY (2008) The activation of NF-κB through Akt-induced FoxO1 phosphorylation during aging and its modulation by calorie restriction. Biogerontology 9:33–47
Kim DH, Perdomo G, Zhang T, Slusher S, Lee S, Phillips BE, Fan Y, Giannoukakis N, Gramignoli R, Strom S, Ringquist S, Dong HH (2011) FoxO6 integrates insulin signaling with gluconeogenesis in the liver. Diabetes 60:2763–2774
Kobayashi Y, Furukawa-Hibi Y, Chen C, Horio Y, Isobe K, Ikeda K, Motoyama N (2005) SIRT1 is critical regulator of FoxO-mediated transcription in response to oxidative stress. Int J Mol Med 16:237–243
Kops GJ, de Ruiter ND, De Vries-Smits AM, Powell DR, Bos JL, Burgering BM (1999) Direct control of the forkhead transcription factor AFX by protein kinase B. Nature 398:630–634
Kops GJ, Dansn TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM (2002) Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 419:316–321
Kouzarides T (2002) Histone methylation in transcriptional control. Curr Opin Genet Dev 12:198–209
Lane MA, Ball SS, Ingram DK, Cutler RG, Engel J, Read V, Roth GS (1995) Diet restriction in rhesus monkeys lowers fasting and glucose stimulated glucoregulatory end points. Am J Physiol 268:941–948
Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villen J, Becker EB, DiBacco S, de la lglesia N, Gygi S, Blackwell TK, Bonni A (2006) A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell 125:987–1001
Li M, Chiu JF, Mossman BT, Fukaqawa NK (2006) Down-regulation of manganese-superoxide dismutase through phosphorylation of FOXO3a by Akt in explanted vascular smooth muscle cells from old rats. J Biol Chem 281:40429–40439
Li Y, Daniel M, Tollefsbol TO (2011) Epigenetic regulation of calorie restriction in aging. BMC Med 9:98
Lin K, Dorman JB, Rodan A, Kenyon C (1997) daf-16: an HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science 278:1319–1322
Lin SJ, Ford E, Haigis M, Liszt G, Guarente L (2004) Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev 18:12–16
Liu ZP, Wang Z, Yanagisawa H, Olson EN (2005) Phenotypic modulation of smooth muscle cells through interaction of FoxO4 and myocardin. Dev Cell 9:261–270
Longo VD, Finch CE (2003) Evolutionary medicine: from dwarf model systems to healthy centenarians? Science 299:1342–1346
Masoro EJ (2005) Overview of caloric restriction and ageing. Mech Ageing Dev 126:913–922
Matsuzaki H, Daitoku H, Hatta M, Tanaka K, Fukamizu A (2003) Insulin-induced phosphorylation of FKHR (Foxo1) targets to proteasomal degradation. Proc Natl Acad Sci USA 100:11285–11290
Matsuzaki H, Daitoku H, Hatta M, Aoyama H, Yoshimochi K, Fukamizu A (2005) Acetylation of Foxo1 alters its DNA-binding ability and sensitivity to phosphorylation. Proc Natl Acad Sci USA 102:11278–11283
Miyauchi H, Minamino T, Tateno K, Kunieda T, Toko H, Komuro I (2004) Akt negatively regulates the in vitro lifespan of human endothelial cells via a p53/p21-dependent pathway. EMBO J 23:212–220
Mohsenzadegan M, Mirshafiey A (2012) The immunopathogenic role of reactive oxygen species in Alzheimer disease. Iran J Allergy Asthma Immunol 11:203–216
Morley JF, Brignull HR, Weyers JJ, Morimoto RI (2002) The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc Natl Acad Sci USA 99:10417–10422
Morris BJ (2005) A forkhead in the road to longevity: the molecular basis of lifespan becomes clearer. J Hypertens 23:1285–1309
Moskalev AA, Smit-McBride Z, Shaposhnikov MV, Plyusnina EN, Zhavoronkov A, Budovsky A, Tacutu R, Fraifeld VE (2012) Gadd45 proteins: relevance to aging, longevity and age-related pathologies. Ageing Res Rev 11:51–66
Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L (2004) Mammalian SIRT1 represses forkhead transcription factors. Cell 116:551–563
Murakami S (2006) Stress resistance in long-lived mouse models. Exp Gerontol 41:1014–1019
Nasrin N, Ogg S, Cahill CM, Biggs W, Nui S, Dore J, Calvo D, Shi Y, Ruvkun G, Alexander-Bridges MC (2000) DAF-16 recruits the CREB-binding protein coactivator complex to the insulin-like growth factor binding protein 1 promoter in HepG2 cells. Proc Natl Acad Sci USA 97:10412–10417
Navab M, Gharavi N, Watson AD (2008) Inflammation and metabolic disorders. Curr Opin Clin Nutr Metab Care 11:459–464
Ni Z, Ebata A, Alipanahiramandi E, Lee SS (2012) Two SET domain containing genes link epigenetic changes and aging in Caenorhabditis elegans. Aging Cell 11:315–325
Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO (2005) Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 310:314–317
Obexer P, Geiger K, Ambros PF, Meister B, Ausserlechner MJ (2007) FKHRL1-mediated expression of Noxa and Bim induces apoptosis via the mitochondria in neuroblastoma cells. Cell Death Differ 14:534–547
Oh SW, Mukhopadhyay A, Svrzikapa N, Jiang F, Davis RJ, Tissenbaum HA (2005) JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc Natl Acad Sci USA 102:4494–4499
Owusu-Ansah E, Banerjee U (2009) Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature 461:537–541
Owusu-Ansah E, Song W, Perrimon N (2013) Muscle mitohormesis promotes longevity via systemic repression of insulin signaling. Cell 155:699–712
Panici JA, Harper JM, Miller RA, Bartke A, Spong A, Masternak MM (2010) Early life growth hormone treatment shortens longevity and decreases cellular stress resistance in long-lived mutant mice. FASEB J 24:5073–5079
Perrot V, Rechler MM (2005) The coactivator p300 directly acetylates the forkhead transcription factor Foxo1 and stimulates Foxo1-induced transcription. Mol Endocrinol 19:2283–2298
Plas DR, Thompson CB (2003) Akt activation promotes degradation of tuberin and FOXO3 via the proteasome. J Biol Chem 278:12361–12366
Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM (2003) Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature 423:550–555
Redman LM, Ravussin E (2011) Caloric restriction in humans: impact on physiological, psychological, and behavioral outcomes. Antioxid Redox Signal 14:275–287
Ribarič S (2012) Diet and aging. Oxid Med Cell Longev 2012:741468
Salih DA, Brunet A (2008) FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr Opin Cell Biol 20:126–136
Salminen A, Kaarniranta K (2012) AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Aging Res Rev 11:230–241
Salminen A, Ojala J, Huuskonen J, Kauppinen A, Suuronen T, Kaarniranta K (2008) Interaction of aging-associated signaling cascades: inhibition of NF-kB signaling by longevity factors FoxOs and SIRT1. Cell Mol Life Sci 65:1049–1058
Senapedis WT, Kennedy CJ, Boyle PM, Silver PA (2011) Whole genome siRNA cell-based screen links mitochondria to Akt signaling network through uncoupling of electron transport chain. Mol Biol Cell 22:1791–1805
Seoane J, Le HV, Shen L, Anderson SA, Massague J (2004) Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell 117:211–223
Sohal RS, Weindruch R (1996) Oxidative stress, caloric restriction, and aging. Science 273:59–63
Sohal RS, Orr WC (2012) The redox stress hypothesis of aging. Free Radic Biol Med 52:539–555
Stankovic M, Mladenovic D, Ninkovic M, Vucevic D, Tomasevic T, Radosavljevic T (2013) Effects of caloric restriction on oxidative stress parameters. Gen Physiol Biophys 32:277–283
Steinberg GR, Kemp BE (2009) AMPK in health and disease. Physiol Rev 89:1025–1078
Stenvinkel P, Karimi M, Johansson S, Axelsson J, Suliman M, Lindholm B, Heimbürger O, Barany P, Alvestrand A, Nordfors L, Qureshi AR, Ekström TJ, Schalling M (2007) Impact of inflammation on epigenetic DNA methylation: a novel risk factor for cardiovascular disease? J Intern Med 261:488–499
Takayama H, Misu H, Iwama H, Chikamoto K, Saito Y, Murao K, Teraguchi A, Lan F, Kikuchi A, Saito R, Tajima N, Shirasaki T, Matsugo S, Miyamoto KI, Kaneko S, Takamura T (2014) Metformin suppresses expression of the selenoprotein P gene via an AMP-activated kinase (AMPK)/FoxO3a pathway in H4IIEC3 hepatocytes. J Biol Chem 289:335–345
Tereshina EV (2009) Metabolic abnormalities as a basis for age-dependent diseases and aging? State of the art. Adv Gerontol 22:129–138
Tsai KL, Sun YJ, Huang CY, Yang JY, Hung MC, Hsiao CD (2007) Crystal structure of the human FOXO3a-DBD/DNA complex suggests the effects of posttranslational modification. Nucleic Acids Res 35:6984–6994
van der Heide LP, Smidt MP (2005) Regulation of FoxO activity by CBP/p300-mediated acetylation. Trends Biochem Sci 30:81–86
van der Heide LP, Hoekman MFM, Smidt MP (2004) The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J 380:297–309
van der Horst A, Burgering BM (2007) Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol 8:440–450
Van der Horst A, Tertoolen LG, de Vries-Smits LM, Frye RA, Medema RH, Burgering BM (2004) FoxO4 is acetylated upon peroxide stress and deacetylated by the longevity protein hSir2 (SIRT1). J Biol Chem 279:28873–28879
van der Horst A, de Vries-Smits AM, Brenkman AB, van Triest MH, van den Broek N, Colland F, Maurice MM, Burqweing BM (2006) FOXO4 transcriptional activity is regulated by monoubiquitination and USP7/HAUSP. Nat Cell Biol 8:1064–1073
Vaquero A, Reinberg D (2009) Calorie restriction and the exercise of chromatin. Genes Dev 23:1849–1869
Wang F, Nguyen M, Qin FX, Tong Q (2007) SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell 6:505–514
Willcox DC, Willcox BJ, Todoriki H, Curb JD, Suzuki M (2006) Caloric restriction and human longevity: what can we learn from the Okinawans? Biogerontology 7:173–177
Xie Q, Hao Y, Tao L, Peng S, Rao C, Chen H, You H, Dong MQ, Yuan Z (2012) Lysine methylation of FOXO3 regulates oxidative stress-induced neuronal cell death. EMBO Rep 13:371–377
Yalcin S, Marinkovic D, Mungamuri SK, Zhang X, Tong W, Sellers R, Ghaffari S (2010) ROS-mediated amplification of Akt/mTOR signaling pathway leads to myeloproliferative syndrome in Foxo3-/- mice. EMBO J 29:4118–4131
Yamagata K, Daitoku H, Takahashi Y, Namiki K, Hisatake K, Kako K, Mukai H, Kasuya Y, Fukamizu A (2008) Arginine methylation of FOXO transcription factors inhibits their phosphorylation by Akt. Mol Cell 32:221–231
Yamamura Y, Lee WL, Inoue K, Ida H, Ito Y (2006) RUNX3 cooperates with FoxO3a to induce apoptosis in gastric cancer cells. J Biol Chem 281:5267–5276
Yamaza H, Komatsu T, Wakita S, Kijogi C, Park S, Hayashi H, Chiba T, Mori R, Furuyama T, Mori N, Shimokawa I (2010) FoxO1 is involved in the antineoplastic effect of calorie restriction. Aging Cell 9:372–382
Yan L, Lavin VA, Moser LR, Cui Q, Kanies C, Yang E (2008) PP2A regulates the pro- apoptotic activity of FOXO1. J Biol Chem 283:7411–7420
Yang Y, Hou H, Haller EM, Nicosia SV, Bai W (2005) Suppression of FOXO1 activity by FHL2 through SIRT1-mediated deacetylation. EMBO J 24:1021–1032
Yellaturu CR, Bhanoori M, Neeli I, Rao GN (2002) N-Ethylmaleimide inhibits platelet-derived growth factor BB-stimulated Akt phosphorylation via activation of protein phosphatase 2A. J Biol Chem 277:40148–40155
Yiu WH, Mead PA, Jun HS, Mansfield BC, Chou JY (2010) Oxidative stress mediates nephropathy in type Ia glycogen storage disease. Lab Invest 90:620–629
Yu BP (1994) Modulation of aging processes by dietary restriction. CRC Press, Boca Raton, pp 1–36
Yu BP (1996) Aging and oxidative stress: modulation by dietary restriction. Free Radic Biol Med 21:651–668
Yu BP (2005) Calorie restriction as a potent anti-aging intervention: suppression of oxidative stress. In: Rattan S (ed) Aging intervention and therapies. World Scientific, Singapore, pp 193–217
Yu BP, Chung HY (2006) The inflammatory process in aging. Rev Clin Gerontol 16:179–187
Zhao X, Gan L, Pan H, Kan D, Majeski M, Adam SA, Unterman TG (2004) Multiple elements regulate nuclear/cytoplasmic shuttling of FOXO1: characterization of phosphorylation- and 14-3-3-dependent and -independent mechanisms. Biochem J 378:839–849
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) funded by the Korean government (MEST) (Grant No. 2009-0083538). This work was carried out with the support of “Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ006522132013)” Rural Development Administration, Republic of Korea. We also take this opportunity to thank the Aging Tissue Bank (Busan, Korea) for supplying research materials.
Conflict of interest
The authors have no conflicts of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, D.H., Park, M.H., Lee, E.K. et al. The roles of FoxOs in modulation of aging by calorie restriction. Biogerontology 16, 1–14 (2015). https://doi.org/10.1007/s10522-014-9519-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-014-9519-y