Abstract
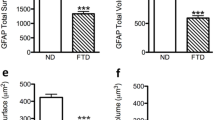
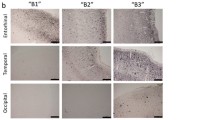
Astrocytes are fundamental for brain physiology and pathology, including Alzheimer’s disease (AD). Among their functions, the maintenance of glutamate balance via the glutamate–glutamine (Glu–Gln) shuttle is critical for both normal cognitive functions and excitotoxicity relevant for AD progression. Astroglial glutamine synthetase (GS), converting glutamate to glutamine, is a key element in the Glu–Gln cycle. The entorhinal cortex (EC) is the brain area earliest affected in human AD. We have recently reported an early astrocytic atrophy in the EC in triple transgenic animal model of AD (3×Tg-AD). Here, we studied and analysed whether the changes in astrocytic morphology coincides with alterations of the Glu–Gln cycle by determining astrocytic GS. We found that the numerical density of GS-immunoreactive (GS-IR) cells as well as GS content (measured by optical density, OD) remained constant between 1 and 12 months of age, independent of the presence of senile plaques. Dual labelling images revealed GS-IR, GFAP-IR, GS/GFAP-IR subsets of astroglia. Despite the evident decrease in GFAP-IR surface and volume, the surface and volume of GS-IR and GS/GFAP-IR cells remained unchanged. Therefore, reduced GFAP presence obvious in the progression of AD from early stages does not impair upon glutamate homeostasis in the EC of 3×Tg-AD mice. Our data also indicate distinct functional populations of astrocytes, which may undergo specific remodelling during AD progression.




Similar content being viewed by others
References
Arsenault D, Julien C, Tremblay C, Calon F (2011) DHA improves cognition and prevents dysfunction of entorhinal cortex neurons in 3 × Tg-AD mice. PLoS ONE 6:e17397
Beauquis J, Pavía P, Pomilio C, Vinuesa A, Podlutskaya N, Galvan V, Saravia F (2013) Environmental enrichment prevents astroglial pathological changes in the hippocampus of APP transgenic mice, model of Alzheimer’s disease. Exp Neurol 239:28–37
Beckstrom H, Julsrud L, Haugeto O, Dewar D, Graham DI, Lehre KP, Storm-Mathisen J, Danbolt NC (1999) Interindividual differences in the levels of the glutamate transporters GLAST and GLT, but no clear correlation with Alzheimer’s disease. J Neurosci Res 55:218–229
Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM (2005) Intraneuronal Abeta causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron 45:675–688
Bliss TV, Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361:31–39
Bouras C, Hof PR, Giannakopoulos P, Michael JP, Morrison JH (1994) Regional distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of elderly patients: a quantitative evaluation of a one-year autopsy population from a geriatric hospital. Cereb Cortex 4:138–150
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259
Braak H, Braak E (1997) Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging 18:351–357
Braak E, Griffing K, Griffing K, Arai K, Bratzke H, Braak H (1991) Neuropathology of Alzheimer’s disease: what is new since A. Alzheimer? Eur Arch Psychiatry Clin Neurosci 249(Suppl 3):14–22
Chvatal A, Anderova M, Hock M, Prajerova I, Neprasova H, Chvatal V, Kirchhoff F, Sykova E (2007) Three-dimensional confocal morphometry reveals structural changes in astrocyte morphology in situ. J Neurosci Res 853:260–271
Colombo JA, Quinn B, Puissant V (2002) Disruption of astroglial interlaminar processes in Alzheimer’s disease. Brain Res Bull 58:235–242
Danbolt NC (2001) Glutamate uptake. Prog Neurobiol 65:1–105
Eliasson C, Sahlgren C, Berthold CH, Stakeberg J, Celis JE, Betsholtz C, Eriksson JE, Pekny M (1999) Intermediate filament protein partnership in astrocytes. J Biol Chem 274:23996–24006
Emsley JG, Macklis JD (2006) Astroglial heterogeneity closely reflects the neuronal-defined anatomy of the adult murine CNS. Neuron Glia Biol 2:175–186
Eng LF, Ghirnikar RS, Lee YL (2000) Glial fibrillary acidic protein: GFAP-thirty-one years (1969-2000). Neurochem Res 25:1439–1451
Hafting T, Fyhn M, Molden S, Moser MB, Moser EI (2005) Microstructure of a spatial map in the entorhinal cortex. Nature 436:801–806
Halassa MM, Haydon PG (2010) Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol 72:335–355
Hill SJ, Barbarese E, McIntosh TK (1996) Regional heterogeneity in the response of astrocytes following traumatic brain injury in the adult rat. J Neuropathol Exp Neurol 55:1221–1229
Hughes EG, Maguire JL, McMinn MT, Scholz RE, Sutherland ML (2004) Loss of glial fibrillary acidic protein results in decreased glutamate transport and inhibition of PKA-induced EAAT2 cell surface trafficking. Brain Res Mol Brain Res 124:114–123
Kerr KM, Agster KL, Furtak SC, Burwell RD (2007) Functional neuroanatomy of the parahippocampal region: the lateral and medial entorhinal areas. Hippocampus 17:697–708
Kulijewicz-Nawrot M, Verkhratsky A, Chvátal A, Syková E, Rodríguez JJ (2012) Astrocytic cytoskeleton atrophy in the medial prefrontal cortex of the triple transgenic mouse model of Alzheimer′s disease. J Ana 221:252–262
Kulijewicz-Nawrot M, Verkhratsky A, Sykova E, Rodríguez JJ (2013) Glutamine synthetase decrease but not GLT-1 glutamate transporter in the prefrontal cortex astroglia of triple transgenic mice model of Alzheimer’s disease. ASN Neuro (in press)
Lau A, Tymianski M (2010) Glutamate receptors, neurotoxicity and neurodegeneration. Pflügers Archiv 460:525–542
Lepekhin EA, Eliasson C, Berthold CH, Berezin V, Bock E, Pekny M (2001) Intermediatefilaments regulate astrocyte motility. J Neurochem 79:617–625
McGaughy J, Koene RA, Eichenbaum H, Hasselmo ME (2005) Cholinergic deafferentation of the entorhinal cortex in rats impairs encoding of novel but not familiar stimuli in a delayed nonmatch-to-sample task. J Neurosci 25:10273–10281
Mishima T, Hirase H (2010) In vivo intracellular recording suggests that gray matter astrocytes in mature cerebral cortex and hippocampus are electrophysiologically homogeneous. J Neurosci 30:3093–3100
Miyake T, Kitamura T (1992) Glutamine synthetase immunoreactivity in two types of mouse brain glial cells. Brain Res 586:53–60
Mohanakrishnan P, Fowler AH, Vonsattel JP, Husain MM, Jolles PR, Liem P, Komoroski RA (1995) An in vitro 1H nuclear magnetic resonance study of the temporoparietal cortex of Alzheimer brains. Exp Brain Res 102:503–510
Muramori F, Kobayashi K, Nakamura I (1998) A quantitative study of neurofibrillary tangles, senile plaques and astrocytes in the hippocampal subdivisions and entorhinal cortex in Alzheimer’s disease, normal controls and non-Alzheimer neuropsychiatric diseases. Psychiatr Clin Neurosci 52:593–599
Naber PA, Lopes da Silva FH, Witter MP (2001) Reciprocal connections between the entorhinal cortex and hippocampal fields CA1 and the subiculum are in register with the projections from CA1 to the subiculum. Hippocampus 11:99–104
Nawashiro H, Brenner M, Fukui S, Shima K, Hallenbeck JM (2000) High susceptibility to cerebral ischemia in GFAP-null mice. J Cereb Blood Flow Metab 20:1040–1044
Noristani HN, Olabarria M, Verkhratsky A, Rodríguez JJ (2010) Serotonin fibre sprouting and increase in serotonin transporter immunoreactivity in the CA1 area of hippocampus in a triple transgenic mouse model of Alzheimer’s disease. Eur J Neurosci 32:71–79
Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM (2003a) Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol Aging 24:1063–1070
Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM (2003b) Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron 39:409–421
Olabarria M, Noristani HN, Verkhratsky A, Rodríguez JJ (2010) Concomitant astroglial atrophy and astrogliosis in a triple transgenic animal model of Alzheimer’s disease. Glia 58:831–838
Olabarria M, Noristani HN, Verkhratsky A, Rodríguez JJ (2011) Age-dependent decrease in glutamine synthetase expression in the hippocampal astroglia of the triple transgenic Alzheimer’s disease mouse model: mechanism for deficient glutamatergic transmission. Mol Neurodegener 6:55
Otani N, Nawashiro H, Fukui S, Ooigawa H, Ohsumi A, Toyooka T, Shima K et al (2006) Enhanced hippocampal neurodegeneration after traumatic or kainate excitotoxicity in GFAP-null mice. J Clin Neurosci 13:934–938
Paxinos G, Franklin K (2004) The mouse Brain in Stereotaxic Coordinates, Compact 2nd Edition. Academic Press, San Diego
Pekny M, Levéen P, Pekna M, Eliasson C, Berthold CH, Westermark B, Betsholtz C (1995) Mice lacking glial fibrillary acidic protein display astrocytes devoid of intermediate filaments but develop and reproduce normally. EMBO J 14:1590–1598
Porchet R, Probst A, Bouras C, Draberova E, Draber P, Riederer BM (2003) Analysis of glial acidic fibrillary protein in the human entorhinal cortex during aging and in Alzheimer’s disease. Proteomics 3:1476–1485
Price JL, Davis PB, Morris JC, White DL (1991) The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer’s disease. Neurobiol Aging 12:295–312
Riedel G, Platt B, Micheau J (2003) Glutamate receptor function in learning and memory. Behav Brain Res 140:1–47
Robbins TW, Murphy ER (2006) Behavioural pharmacology: 40 + years of progress, with a focus on glutamate receptors and cognition. Trends Pharmacol Sci 27:141–148
Robinson SR (2000) Neuronal expression of glutamine synthetase in Alzheimer’s disease indicates a profound impairment of metabolic interactions with astrocytes. Neurochem Int 36:471–482
Robinson SR (2001) Changes in the cellular distribution of glutamine synthetase in Alzheimer’s disease. J Neurosci Res 66:972–980
Rodríguez JJ, Jones VC, Tabuchi M, Allan SM, Knight EM, LaFerla FM, Oddo S, Verkhratsky A (2008) Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of Alzheimer’s disease. PLoS ONE 3:e2935
Rodríguez JJ, Jones VC, Verkhratsky A (2009a) Impaired cell proliferation in the subventricular zone in an Alzheimer’s disease model. NeuroReport 20:907–912
Rodríguez JJ, Olabarria M, Chvatal A, Verkhratsky A (2009b) Astroglia in dementia and Alzheimer’s disease. Cell Death Differ 16:378–385
Rothestein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF (1996) Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16:675–686
Simpson JE, Ince PG, Shaw PJ, Heath PR, Raman R, Garwood CJ, Gelsthorpe C, Baxter L, Forster G, Matthews FE, Brayne C, Wharton SB (2011) Microarray analysis of the astrocyte transcriptome in the aging brain: relationship to Alzheimer’s pathology and APOE genotype. Neurobiol Aging 32:1795–1807
Suzuki WA, Amaral DG (1994) Topographic organization of the reciprocal connections between the monkey entorhinal cortex and the perirhinal and parahippocampal cortices. J Neurosci 14:1856–1877
Tamamaki N, Nojyo Y (1993) Projection of the entorhinal layer II neurons in the rat as revealed by intracellular pressure-injection of neurobiotin. Hippocampus 3:471–480
Verkhratsky A, Olabarria M, Noristani HN, Yeh CY, Rodríguez JJ (2010) Astrocytes in Alzheimer’s disease. Neurotherapeutics 7:399–412
Verkhratsky A, Rodríguez JJ, Papura V (2012) Neurotransmitters and integration in neuronal-astroglial network. Neurochem Res 37:2326–2338
Walton HS, Dodd PR (2007) Glutamate-glutamine cycling in Alzheimer’s disease. Neurochem Int 50:1052–1066
Wilcock DM, Vitek MP, Colton CA (2009) Vascular amyloid alters astrocytic water and potassium channels in mouse models and humans with Alzheimer’s disease. Neuroscience 159:1055–1069
Wilhelmsson U, Li L, Pekna M, Berthold C-H, Blom S, Eliasson C, Renner O, Bushong E, Ellisman M, Morgan TE, Pekny M (2004) Absence of glial fibrillary acidic protein and vimentin prevents hypertrophy of astrocytic processes and improves post-traumatic regeneration. J Neurosci 24:5016–5021
Witter MP, Van Hoesen GW, Amaral DG (1989) Topographical organization of the entorhinal projection to the dentate gyrus of the monkey. J Neurosci 9:216–228
Yeh CY, Vadhwana B, Verkhratsky A, Rodríguez JJ (2011) Early astrocytic atrophy in the entorhinal cortex of a triple transgenic animal model of Alzheimer’s disease. ASN Neuro 3:271–279
Zhang Y, Barres BA (2010) Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr Opin Neurobiol 20:588–594
Acknowledgments
This study was supported by an Alzheimer’s Research Trust Programme Grant [ART/PG2004A/1] to JJR and AV, grants from the Grant Agency of the Czech Republic to JJR ([GACR 309/09/1696], to ES and JJR [GACR 304/11/0184] and to AV [GACR 305/08/1381, GACR 305/08/1384]) and by the Welcome Trust.. Support from the Spanish Government, Plan Nacional de I+D+I 2008-2011 and ISCIII- Subdirección General de Evaluación y Fomento de la Investigación [PI10/02738] co-financed by FEDER to JJR and AV and the Government of the Basque Country [AE-2010-1-28, AEGV10/16, GV-2011111020] are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yeh, CY., Verkhratsky, A., Terzieva, S. et al. Glutamine synthetase in astrocytes from entorhinal cortex of the triple transgenic animal model of Alzheimer’s disease is not affected by pathological progression. Biogerontology 14, 777–787 (2013). https://doi.org/10.1007/s10522-013-9456-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-013-9456-1