Abstract
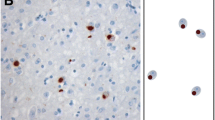
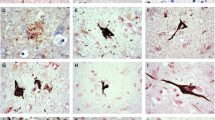
Hyperphosphorylation of microtubule associated protein tau had limited studies in Alzheimer’s disease (AD) brainstem. We compared the distribution and number of neurons with hyperphosphorylated tau in two age groups of AD brainstems with mean ages of 65.4 ± 5.7 and 91.1 ± 6.4 years. The degree of co-localization of hyperphosphorylated tau positive cells with either cleaved caspase-3 or cleaved caspase-6 was also quantified. Results showed hyperphosphorylated tau mainly occurred in hypoglossal, dorsal motor vagal, trigeminal sensory/motor nuclei as well as in dorsal raphe, locus coeruleus and substantia nigra. Older AD brainstem consistently had higher density of hyperphosphorylated tau cells. Up to 70% of tau positive cells also displayed either cleaved caspase-3 or caspase-6, and the number of co-localized tau cells in each caspase subfamily group was always higher in older aged group. Some hyperphosphorylated tau cells with cleaved caspases had TUNEL positive nuclei. These findings suggest that these latter cells went through the apoptotic process or DNA fragmentation.





Similar content being viewed by others
References
Abercrombie M, Johnson ML (1946) Quantitative histology of Wallerian degeneration: I. Nuclear population in rabbit sciatic nerve. J Anat 80:37–50
Aksenov MY, Tucker HM, Nair P et al (1998) The expression of key oxidative stress-handling genes in different brain regions in Alzheimer’s disease. J Mol Neurosci 11:151–164. doi:10.1385/JMN:11:2:151
Albrecht S, Bourdeau M, Bennett D et al (2007) Activation of caspase-6 in aging and mild cognitive impairment. Am J Pathol 170:1200–1209. doi:10.2353/ajpath.2007.060974
Aletrino MA, Vogels OJ, Van Domburg PH et al (1992) Cell loss in the nucleus raphes dorsalis in Alzheimer’s disease. Neurobiol Aging 13:461–468. doi:10.1016/0197-4580(92)90073-7
Alonso AC, Grundke-Iqbal I, Iqbal K (1996) Alzheimer’s disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat Med 2:783–787. doi:10.1038/nm0796-783
Alzheimer A (1911) Über eigenartige Krankheitsfälle des späteren Alters. Zeitschrift für die gesamte. Neurol Psychiatr (Bucur) 4:356–385
Andorfer C, Acker CM, Kress Y et al (2005) Cell-cycle reentry and cell death in transgenic mice expressing nonmutant human tau isoforms. J Neurosci 25:5446–5454. doi:10.1523/JNEUROSCI.4637-04.2005
Arendt T, Bruckner MK, Bigl V et al (1995) Dendritic reorganisation in the basal forebrain under degenerative conditions and its defects in Alzheimer’s disease. III. The basal forebrain compared with other subcortical areas. J Comp Neurol 351:223–246. doi:10.1002/cne.903510204
Arnold SE, Hyman BT, Flory J et al (1991) The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb Cortex 1:103–116. doi:10.1093/cercor/1.1.103
Attems J, Quass M, Jellinger KA (2007) Tau and alpha-synuclein brainstem pathology in Alzheimer disease: relation with extrapyramidal signs. Acta Neuropathol 113:53–62. doi:10.1007/s00401-006-0146-9
Avila J, Pérez M, Lim F et al (2004) Tau in neurodegenerative diseases: tau phosphorylation and assembly. Neurotox Res 6:477–482
Ballard CG, Jacoby R, Del Ser T et al (2004) Neuropathological substrates of psychiatric symptoms in prospectively studied patients with autopsy-confirmed dementia with lewy bodies. Am J Psychiatry 161:843–849. doi:10.1176/appi.ajp.161.5.843
Bancher C, Brunner C, Lassmann H et al (1989) Tau and ubiquitin immunoreactivity at different stages of formation of Alzheimer neurofibrillary tangles. Prog Clin Biol Res 317:837–848
Berger AB, Witte MD, Denault JB et al (2006) Identification of early intermediates of caspase activation using selective inhibitors and activity-based probes. Mol Cell 23:509–521. doi:10.1016/j.molcel.2006.06.021
Bondareff W, Mountjoy CQ, Roth M et al (1989) Neurofibrillary degeneration and neuronal loss in Alzheimer’s disease. Neurobiol Aging 10:709–715. doi:10.1016/0197-4580(89)90007-9
Braak H, Braak E (1995) Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging 16:271–284. doi:10.1016/0197-4580(95)00021-6
Braak H, Braak E (1998) Evolution of neuronal changes in the course of Alzheimer’s disease. J Neural Transm Suppl 53:127–140
Braak E, Braak H, Mandelkow EM (1994) A sequence of cytoskeleton changes related to the formation of neurofibrillary tangles and neuropil threads. Acta Neuropathol 87:554–567. doi:10.1007/BF00293315
Braak H, Alafuzoff I, Arzberger T et al (2006) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112:389–404. doi:10.1007/s00401-006-0127-z
Brodal A (1981) Neurological anatomy in relation to clinical medicine. Oxford University Press, New York
Burns JM, Galvin JE, Roe CM et al (2005) The pathology of the substantia nigra in Alzheimer disease with extrapyramidal signs. Neurology 64:1397–1403
Chen F, Chang R, Trivedi M et al (2003) Caspase proteolysis of desmin produces a dominant-negative inhibitor of intermediate filaments and promotes apoptosis. J Biol Chem 278:6848–6853. doi:10.1074/jbc.M212021200
Cowling V, Downward J (2002) Caspase-6 is the direct activator of caspase-8 in the cytochrome c-induced apoptosis pathway: absolute requirement for removal of caspase-6 prodomain. Cell Death Differ 9:1046–1056. doi:10.1038/sj.cdd.4401065
Crowther RA, Goedert M (2000) Abnormal tau-containing filaments in neurodegenerative diseases. J Struct Biol 130:271–279. doi:10.1006/jsbi.2000.4270
Curcio CA, Kemper T (1984) Nucleus raphe dorsalis in dementia of the Alzheimer type: neurofibrillary changes and neuronal packing density. J Neuropathol Exp Neurol 43:359–368. doi:10.1097/00005072-198407000-00001
Dom R, Lammens M, Saedeleer JD et al (1989) Cytometrical and immunocytochemical investigation of brain nuclei in dementia. Prog Clin Biol Res 317:375–381
Forman MS, Lee VM, Trojanowski JQ (2000) New insights into genetic and molecular mechanisms of brain degeneration in tauopathies. J Chem Neuroanat 20:225–244. doi:10.1016/S0891-0618(00)00100-9
Gamblin TC, Chen F, Zambrano A et al (2003) Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc Natl Acad Sci USA 100:10032–10037. doi:10.1073/pnas.1630428100
Grudzien A, Shaw P, Weintraub S et al (2007) Locus coeruleus neurofibrillary degeneration in aging, mild cognitive impairment and early Alzheimer’s disease. Neurobiol Aging 28:327–335. doi:10.1016/j.neurobiolaging.2006.02.007
Grundke-Iqbal I, Iqbal K, Tung YC et al (1986) Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA 83:4913–4917. doi:10.1073/pnas.83.13.4913
Guo H, Albrecht S, Bourdeau M et al (2004) Active caspase-6 and caspase-6-cleaved tau in neuropil threads, neuritic plaques, and neurofibrillary tangles of Alzheimer’s disease. Am J Pathol 165:523–531
Horowitz PM, Patterson KR, Guillozet-Bongaarts AL et al (2004) Early N-terminal changes and caspase-6 cleavage of tau in Alzheimer’s disease. J Neurosci 24:7895–7902. doi:10.1523/JNEUROSCI.1988-04.2004
Imai Y, Ibata I, Ito D et al (1996) A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem Biophys Res Commun 224:855–862. doi:10.1006/bbrc.1996.1112
Iqbal K, Alonso AC, Gong CX et al (1998) Mechanisms of neurofibrillary degeneration and the formation of neurofibrillary tangles. J Neural Transm Suppl 53:169–180
Iqbal K, Alonso Adel C, Chen S et al (2005) Tau pathology in Alzheimer disease and other tauopathies. Biochim Biophys Acta 1739:198–210
Ishii T (1966) Distribution of Alzheimer’s neurofibrillary changes in the brain stem and hypothalamus of senile dementia. Acta Neuropathol 6:181–187. doi:10.1007/BF00686763
Ishino H, Otsuki S (1975) Frequency of Alzheimer’s neurofibrillary tangles in the basal ganglia and brain-stem in Alzheimer’s disease, senile dementia and the aged. Folia Psychiatr Neurol Jpn 29:279–287
Jellinger KA, Stadelmann C (2000) Mechanisms of cell death in neurodegenerative disorders. J Neural Transm Suppl 59:95–114
Johnson GV, Stoothoff WH (2004) Tau phosphorylation in neuronal cell function and dysfunction. J Cell Sci 117:5721–5729. doi:10.1242/jcs.01558
Kimura T, Ono T, Takamatsu J et al (1996) Sequential changes of tau-site-specific phosphorylation during development of paired helical filaments. Dementia 7:177–181. doi:10.1159/000106875
Kosaka K, Iizuka R, Mizutani Y et al (1981) Striatonigral degeneration combined with Alzheimer’s disease. Acta Neuropathol 54:253–256. doi:10.1007/BF00687749
Lassmann H, Bancher C, Breitschopf H et al (1995) Cell death in Alzheimer’s disease evaluated by DNA fragmentation in situ. Acta Neuropathol 89:35–41. doi:10.1007/BF00294257
LeBlanc AC (2005) The role of apoptotic pathways in Alzheimer’s disease neurodegeneration and cell death. Curr Alzheimer Res 2:389–402. doi:10.2174/156720505774330573
Lee HG, Perry G, Moreira PI et al (2005) Tau phosphorylation in Alzheimer’s disease: pathogen or protector? Trends Mol Med 11:164–169. doi:10.1016/j.molmed.2005.02.008
Li WP, Chan WY, Lai HW et al (1997) Terminal dUTP nick end labeling (TUNEL) positive cells in the different regions of the brain in normal aging and Alzheimer patients. J Mol Neurosci 8:75–82. doi:10.1007/BF02736774
Lindwall G, Cole RD (1984) Phosphorylation affects the ability of tau protein to promote microtubule assembly. J Biol Chem 259:5301–5305
Love S, Wilcock GK, Matthews SM (1996) No correlation between nigral degeneration and striatal plaques in Alzheimer’s disease. Acta Neuropathol 91:432–436. doi:10.1007/s004010050447
Luna LG (1968) Manual of histologic staining methods of the Armed Forces Institute of Pathology, 3rd edn. Blakiston Division, McGraw Hill, NY
Maurage CA, Sergeant N, Ruchoux MM et al (2003) Phosphorylated serine 199 of microtubule-associated protein tau is a neuronal epitope abundantly expressed in youth and an early marker of tau pathology. Acta Neuropathol 105:89–97
Murayama S, Saito Y (2004) Neuropathological diagnostic criteria for Alzheimer’s disease. Neuropathology 24:254–260. doi:10.1111/j.1440-1789.2004.00571.x
Neumann PJ, Araki SS, Arcelus A et al (2001) Measuring Alzheimer’s disease progression with transition probabilities: estimates from CERAD. Neurology 57:943–944
Parvizi J, Van Hoesen GW, Damasio A (2000) Selective pathological changes of the periaqueductal gray matter in Alzheimer’s disease. Ann Neurol 48:344–353. doi:10.1002/1531-8249(200009)48:3<344::AID-ANA9>3.0.CO;2-S
Parvizi J, Van Hoesen GW, Damasio A (2001) The selective vulnerability of brainstem nuclei to Alzheimer’s disease. Ann Neurol 49:53–66. doi:10.1002/1531-8249(200101)49:1<53::AID-ANA30>3.0.CO;2-Q
Raynaud F, Marcilhac A (2006) Implication of calpain in neuronal apoptosis. A possible regulation of Alzheimer’s disease. FEBS J 273:3437–3443. doi:10.1111/j.1742-4658.2006.05352.x
Schwab C, Steele JC, McGeer PL (1998) Pyramidal neuron loss is matched by ghost tangle increase in Guam Parkinsonism-dementia hippocampus. Acta Neuropathol 96:409–416. doi:10.1007/s004010050912
Schwab C, Schulzer M, Steele JC et al (1999) On the survival time of a tangled neuron in the hippocampal CA4 region in Parkinsonism dementia complex of Guam. Neurobiol Aging 20:57–63. doi:10.1016/S0197-4580(99)00005-6
Shahani N, Brandt R (2002) Functions and malfunctions of the tau proteins. Cell Mol Life Sci 59:1668–1680. doi:10.1007/PL00012495
Spillantini MG, Goedert M (1998) Tau protein pathology in neurodegenerative diseases. Trends Neurosci 21:428–433. doi:10.1016/S0166-2236(98)01337-X
Sugaya K, Reeves M, McKinney M (1997) Topographic associations between DNA fragmentation and Alzheimer’s disease neuropathology in the hippocampus. Neurochem Int 31:275–281. doi:10.1016/S0197-0186(96)00158-1
Woolf NJ, Jacobs RW, Butcher LL (1989) The pontomesencephalotegmental cholinergic system does not degenerate in Alzheimer’s disease. Neurosci Lett 96:277–282. doi:10.1016/0304-3940(89)90391-1
Yamamoto T, Hirano A (1985) Nucleus raphe dorsalis in Alzheimer’s disease: neurofibrillary tangles and loss of large neurons. Ann Neurol 17:573–577. doi:10.1002/ana.410170608
Yamamoto H, Saitoh Y, Fukunaga K et al (1988) Dephosphorylation of microtubule proteins by brain protein phosphatases 1 and 2A, and its effect on microtubule assembly. J Neurochem 50:1614–1623. doi:10.1111/j.1471-4159.1988.tb03051.x
Zarow C, Lyness SA, Mortimer JA et al (2003) Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch Neurol 60:337–341. doi:10.1001/archneur.60.3.337
Zee LG (1964) Quantitative biological techniques, 1st edn. Scientific Press, Beijing, 252 pp
Zhang Y, Goodyer C, LeBlanc A (2000) Selective and protracted apoptosis in human primary neurons microinjected with active caspase-3, -6, -7, and -8. J Neurosci 20:8384–8389
Zhou XW, Li X, Bjorkdahl C et al (2006) Assessments of the accumulation severities of amyloid beta-protein and hyperphosphorylated tau in the medial temporal cortex of control and Alzheimer’s brains. Neurobiol Dis 22:657–668. doi:10.1016/j.nbd.2006.01.006
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wai, M.S.M., Liang, Y., Shi, C. et al. Co-localization of hyperphosphorylated tau and caspases in the brainstem of Alzheimer’s disease patients. Biogerontology 10, 457–469 (2009). https://doi.org/10.1007/s10522-008-9189-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-008-9189-8