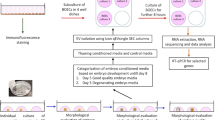
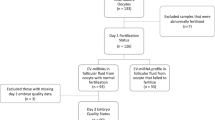
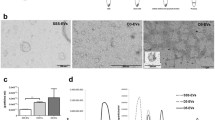
We studied the influence of extracellular vesicles from the follicular fluid of a young donor on gene expression (MKI67, MYBL2, CCNB1, CCND1, CCNE1, CALM2, BAX, NDRG1, TP53I3, VEGF, VCAN, HAS2, CTSL2, PIBF1, RPL37, PFKP, GPX3, and AQP3) in embryos of women of different ages. According to nanoparticle tracking analysis data, the concentration of extracellular vesicles was 3.75±0.47×1011 particles/ml and the mean particle size was 138.78±9.90 nm. During co-culturing of the follicular fluid extracellular vesicles with blastocysts of young women, we observed significantly increased expression of mRNA for genes CTSL2, CCND1, CCNE1, VEGF and reduced expression of BAX gene mRNA in comparison with embryos in women of late reproductive age. We hypothesized that addition of extracellular vesicles of the oocyte follicular fluid from a young donor to the culture medium of embryos could slow down apoptosis process typical of blastocyst cells in women above 36 years.
Similar content being viewed by others
References
Wang X, Wang L, Xiang W. Mechanisms of ovarian aging in women: a review. J. Ovarian Res. 2023;16(1):67. doi: https://doi.org/10.1186/s13048-023-01151-z
Fan W, Qi Y, Wang Y, Yan H, Li X, Zhang Y. Messenger roles of extracellular vesicles during fertilization of gametes, development and implantation: Recent advances. Front. Cell Dev. Biol. 2023;10:1079387. doi: https://doi.org/10.3389/fcell.2022.1079387
Manni G, Buratta S, Pallotta MT, Chiasserini D, Di Michele A, Emiliani C, Giovagnoli S, Pascucci L, Romani R, Bellezza I, Urbanelli L, Fallarino F. Extracellular vesicles in aging: an emerging hallmark? Cells. 2023;12(4):527. doi: https://doi.org/10.3390/cells12040527
Sysoeva AP, Makarova NP, Silachev DN, Lobanova NN, Shevtsova YA, Bragina EE, Kalinina EA, Sukhikh GT. Influence of extracellular vesicles of the follicular fluid on morphofunctional characteristics of human sperm. Bull. Exp. Biol. Med. 2021;172(2):254-262. doi: https://doi.org/10.1007/s10517-021-05372-4
Sysoeva AP, Nepsha OS, Makarova NP, Silachev DN, Lobanova NN, Timofeeva AV, Shevtsova YA, Bragina EE, Kalinina EA. Influence of extracellular vesicles from the follicular fluid of young women and women of advanced maternal age with different miRNA profiles on sperm functional properties. Bull. Exp. Biol. Med. 2022;173(4):560-568. doi: https://doi.org/10.1007/s10517-022-05589-x
Gabryś J, Kij-Mitka B, Sawicki S, Kochan J, Nowak A, Łojko J, Karnas E, Bugno-Poniewierska M. Extracellular vesicles from follicular fluid may improve the nuclear maturation rate of in vitro matured mare oocytes. Theriogenology. 2022;188:116-124. doi: https://doi.org/10.1016/j.theriogenology.2022.05.022
da Silveira JC, Andrade GM, Del Collado M, Sampaio RV, Sangalli JR, Silva LA, Pinaffi FVL, Jardim IB, Cesar MC, Nogueira MFG, Cesar ASM, Coutinho LL, Pereira RW, Perecin F, Meirelles FV. Supplementation with small-extracellular vesicles from ovarian follicular fluid during in vitro production modulates bovine embryo development. PLoS One. 2017;12(6):e0179451. doi: https://doi.org/10.1371/journal.pone.0179451
Kenigsberg S, Wyse BA, Librach CL, da Silveira JC. Protocol for exosome isolation from small volume of ovarian follicular fluid: evaluation of ultracentrifugation and commercial kits. Methods Mol. Biol. 2017;1660:321-341. doi: https://doi.org/10.1007/978-1-4939-7253-1_26
Soares M, Pinto MM, Nobre RJ, de Almeida LP, da Graça Rasteiro M, Almeida-Santos T, Ramalho-Santos J, Sousa AP. Isolation of extracellular vesicles from human follicular fluid: size-exclusion chromatography versus ultracentrifugation. Biomolecules. 2023;13(2):278. doi: https://doi.org/10.3390/biom13020278
Lim S, Kaldis P. Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development. 2013;140(15):3079-3093. doi: https://doi.org/10.1242/dev.091744
Raes A, Wydooghe E, Pavani KC, Bogado Pascottini O, Van Steendam K, Dhaenens M, Boel A, Heras S, Heindryckx B, Peelman L, Deforce D, Van Nieuwerburgh F, Opsomer G, Van Soom A, Smits K. Cathepsin-L secreted by high-quality bovine embryos exerts an embryotrophic effect in vitro. Int. J. Mol. Sci. 2023;24(7):6563. doi: https://doi.org/10.3390/ijms24076563
Khurana P, Smyth NR, Sheth B, Velazquez MA, Eckert JJ, Fleming TP. Advanced maternal age perturbs mouse embryo development and alters the phenotype of derived embryonic stem cells. J. Dev. Orig. Health Dis. 2022;13(3):395-405. doi: https://doi.org/10.1017/S2040174421000325
Kawai K, Harada T, Ishikawa T, Sugiyama R, Kawamura T, Yoshida A, Tsutsumi O, Ishino F, Kubota T, Kohda T. Parental age and gene expression profiles in individual human blastocysts. Sci. Rep. 2018;8(1):2380. doi: https://doi.org/10.1038/s41598-018-20614-8
McCallie BR, Parks JC, Trahan GD, Jones KL, Coate BD, Griffin DK, Schoolcraft WB, Katz-Jaffe MG. Compromised global embryonic transcriptome associated with advanced maternal age. J. Assist. Reprod. Genet. 2019;36(5):915-924. doi: https://doi.org/10.1007/s10815-019-01438-5
Ezoe K, Miki T, Akaike H, Shimazaki K, Takahashi T, Tanimura Y, Amagai A, Sawado A, Mogi M, Kaneko S, Ueno S, Coticchio G, Cimadomo D, Borini A, Rienzi L, Kato K. Maternal age affects pronuclear and chromatin dynamics, morula compaction and cell polarity, and blastulation of human embryos. Hum. Reprod. 2023;38(3):387-399. doi: https://doi.org/10.1093/humrep/dead001
Chaudhry SR, Lopes J, Levin NK, Kalpage H, Tainsky MA. Germline mutations in apoptosis pathway genes in ovarian cancer; the functional role of a TP53I3 (PIG3) variant in ROS production and DNA repair. Cell Death Discov. 2021;7(1):62. doi: https://doi.org/10.1038/s41420-021-00442-y
Jiang XD, Liu Y, Wu JF, Gong SN, Ma Y, Zi XD. Regulation of proliferation, apoptosis, hormone secretion and gene expression by acetyl-L-carnitine in yak (Bos grunniens) granulosa cells. Theriogenology. 2023;203:61-68. doi: https://doi.org/10.1016/j.theriogenology.2023.03.016
Diez-Fraile A, Lammens T, Tilleman K, Witkowski W, Verhasselt B, De Sutter P, Benoit Y, Espeel M, D’Herde K. Age-associated differential microRNA levels in human follicular fluid reveal pathways potentially determining fertility and success of in vitro fertilization. Hum. Fertil. (Camb). 2014;17(2):90-98. doi: https://doi.org/10.3109/14647273.2014.897006
Battaglia R, Musumeci P, Ragusa M, Barbagallo D, Scalia M, Zimbone M, Lo Faro JM, Borzì P, Scollo P, Purrello M, Vento EM, Di Pietro C. Ovarian aging increases small extracellular vesicle CD81+ release in human follicular fluid and influences miRNA profiles. Aging (Albany NY). 2020;12(12):12 324-12 341. doi: https://doi.org/10.18632/aging.103441
Fang X, Tanga BM, Bang S, Seo C, Kim H, Saadeldin IM, Lee S, Cho J. Oviduct epithelial cell-derived extracellular vesicles improve porcine trophoblast outgrowth. Vet. Sci. 2022;9(11):609. doi: https://doi.org/10.3390/vetsci9110609
Qu P, Luo S, Du Y, Zhang Y, Song X, Yuan X, Lin Z, Li Y, Liu E. Extracellular vesicles and melatonin benefit embryonic develop by regulating reactive oxygen species and 5-methylcytosine. J. Pineal Res. 2020;68(3):e12635. doi: https://doi.org/10.1111/jpi.12635
Tsukazawa KS, Li L, Tse WKF. 2,4-dichlorophenol exposure induces lipid accumulation and reactive oxygen species formation in zebrafish embryos. Ecotoxicol. Environ. Saf. 2022;230:113133. doi: https://doi.org/10.1016/j.ecoenv.2021.113133
Qu P, Zhao Y, Wang R, Zhang Y, Li L, Fan J, Liu E. Extracellular vesicles derived from donor oviduct fluid improved birth rates after embryo transfer in mice. Reprod. Fertil. Dev. 2019;31(2):324-332. doi: https://doi.org/10.1071/RD18203
Sidrat T, Khan AA, Joo MD, Wei Y, Lee KL, Xu L, Kong IK. Bovine oviduct epithelial cell-derived culture media and exosomes improve mitochondrial health by restoring metabolic flux during pre-implantation development. Int. J. Mol. Sci. 2020;21(20):7589. doi: https://doi.org/10.3390/ijms21207589
Fang X, Bang S, Tanga BM, Seo C, Zhou D, Seong G, Saadeldin IM, Lee S, Cui XS, Cho J. Oviduct epithelial cell-derived extracellular vesicles promote the developmental competence of IVF porcine embryos. Mol. Med. Rep. 2023;27(6):122. doi: https://doi.org/10.3892/mmr.2023.13009
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Kletochnye Tekhnologii v Biologii i Meditsine, No. 4, pp. 233-241, December, 2023
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nepsha, O.S., Burmenskaya, O.V., Akhmedova, Z.F. et al. Changes in the Transcription of Proliferation- and Apoptosis-Related Genes in Embryos in Women of Different Ages under the Influence of Extracellular Vesicles from Donor Follicular Fluid In Vitro. Bull Exp Biol Med 176, 658–665 (2024). https://doi.org/10.1007/s10517-024-06087-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-024-06087-y