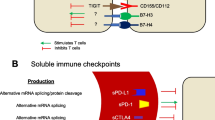
The content of the soluble forms of immune checkpoint components sPD-1, sPD-L1 in blood serum, and sB7-H3, sCD314, sULBP1, sHLA-G in blood plasma of 30 melanoma patients receiving immunotherapy with anti-PD-1 antibodies (nivolumab, pembrolisumab) was measured before and in 4 and 8 weeks after the start of immunotherapy. The control group comprised 70 practically healthy donors. Standard immunoassay kits were used. In melanoma patients, the levels of sPD-L1 and sB7-H3 were significantly higher than in the control group (p<0001), sPD-1 level did not differ from the control, while sCD314 and sHLA-G levels were insignificantly decreased. During therapy, opposite changes in the levels of markers in individual patients were observed, and frequently after the initial increase (or decrease) after the first 4 weeks normalization did occur in the further 4 weeks. No statistically significant associations between the initial levels of markers and direction of their changes during treatment were found, but some trends indicating to the potential benefits from assessment of soluble forms of immune checkpoint proteins for evaluation and monitoring of the efficiency of the therapy with immune checkpoint blockers were revealed: significant decrease of sB7-H3 and sPD-1 levels in the course of treatment, higher initial sPD-1 level in patients with future progression than in those with stabilization or partial effect, and lower progression frequency in patients with increasing sPD-1 and sPD-L1 levels than in those with decreasing markers levels.
Similar content being viewed by others
References
Kushlinskii NE, Fridman MV, Morozov AA, Gershtein ES, Kadagidze ZG, Matveev VB. Modern approaches to kidney cancer immunotherapy. Onkourologiya. 2018;14(2):54-67. Russian. https://doi.org/10.17650/1726-9776-2018-14-2-54-67
Alferov AA, Efimova MM, Kuzmin YuB, Kuznetsov IN, Gershtein ES, Kushlinskii NE. Key immune checkpoints and their inhibitors in the therapy of bone tumors. Part 2. Additional targets for immunotherapy of bone tumors and markers of its efficiency. Tekhnol. Zhivykh Sistem. 2021;18(1):18-31. Russian. https://doi.org/10.18127/j20700997-202101-02
Huang FX, Wu JW Cheng XQ, Wang JH, Wen XZ, Li JJ, Zhang Q, Jiang H, Ding QY, Zhu XF, Zhang XS, Ding Y, Li DD. HHLA2 predicts improved prognosis of anti-PD-1/PD-L1 immunotherapy in patients with melanoma. Front. Immunol. 2022;13:902167. https://doi.org/10.3389/fimmu.2022.902167
Yu J, Wu X, Song J, Zhao Y, Li H, Luo M, Liu X. Loss of MHC-I antigen presentation correlated with immune checkpoint blockade tolerance in MAPK inhibitor-resistant melanoma. Front. Pharmacol. 2022;13:928226. https://doi.org/10.3389/fphar.2022.928226
Gellrich FF, Schmitz M, Beissert S, Meier F. Anti-PD-1 and Novel Combinations in the Treatment of Melanoma-An Update. J. Clin. Med. 2020;9(1):223. https://doi.org/10.3390/jcm9010223
Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer. 2016;16(5):275-287. https://doi.org/10.1038/nrc.2016.36
Ning B, Liu Y, Wang M, Li Y, Xu T, Wei Y. The Predictive Value of Tumor Mutation Burden on Clinical Efficacy of Immune Checkpoint Inhibitors in Melanoma: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2022;13:748674. https://doi.org/10.3389/fphar.2022.748674
Wei W, Xu B, Wang Y, Wu C, Jiang J, Wu C. Prognostic significance of circulating soluble programmed death ligand-1 in patients with solid tumors: A meta-analysis. Medicine (Baltimore). 2018;97(3):e9617. https://doi.org/10.1097/MD.0000000000009617
Zhou J, Mahoney KM, Giobbie-Hurder A, Zhao F, Lee S, Liao X, Rodig S, Li J, Wu X, Butterfield LH, Piesche M, Manos MP, Eastman LM, Dranoff G, Freeman GJ, Hodi FS. Soluble PD-L1 as a Biomarker in Malignant Melanoma Treated with Checkpoint Blockade. Cancer Immunol. Res. 2017;5(6):480-492. https://doi.org/10.1158/2326-6066.CIR-16-0329.
Carlino MS, Larkin J, Long GV. Immune checkpoint inhibitors in melanoma. Lancet. 2021;398:1002-1014. https://doi.org/10.1016/S0140-6736(21)01206-X
Bersanelli M, Petrelli F, Buti S, Stanganelli I. Immune checkpoint inhibitors in adjuvant setting after radical resection of melanoma: a meta-analysis of the pivotal trials. Hum. Vaccin. Immunother. 2022;18(3):1902723. https://doi.org/10.1080/21645515.2021
Aroldi F, Middleton MR. Long-Term Outcomes of Immune Checkpoint Inhibition in Metastatic Melanoma. Am. J. Clin. Dermatol. 2022;23(3):331-338. https://doi.org/10.1007/s40257-022-00681-4
Nebhan CA, Johnson DB. Predictive biomarkers of response to immune checkpoint inhibitors in melanoma. Expert Rev. Anticancer Ther. 2020;20(2):137-145. https://doi.org/10.1080/14737140.2020.1724539
Singh L, Singh MK, Kenney MC, Jager MJ, Rizvi MA, Meel R, Lomi N, Bakhshi S, Sen S, Kashyap S. Prognostic significance of PD-1/PD-L1 expression in uveal melanoma: correlation with tumor-infiltrating lymphocytes and clinicopathological parameters. Cancer Immunol. Immunother. 2021;70(5):1291-1303. https://doi.org/10.1007/s00262-020-02773-8
Yuan B, Miao L, Mei D, Li L, Zhou Q, Dong D, Wang S, Zhu X, Xu S. Value of a Signature of Immune-Related Genes in Predicting the Prognosis of Melanoma and Its Responses to Immune Checkpoint Blocker Therapies. Comput. Math. Methods Med. 2022;2022:9633416. https://doi.org/10.1155/2022/9633416
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Byulleten’ Eksperimental’noi Biologii i Meditsiny, Vol. 175, No. 4, pp. 482-487, April, 2023
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gershtein, E.S., Mochalova, A.S., Korotkova, E.A. et al. Dynamics of Soluble Forms of the Immune Checkpoint Components PD-1/PD-L1/B7-H3, CD314/ULBP1, and HLA-G in Peripheral Blood of Melanoma Patients Receiving Blockers of Programmed Cell Death Protein PD-1. Bull Exp Biol Med 175, 481–486 (2023). https://doi.org/10.1007/s10517-023-05891-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-023-05891-2