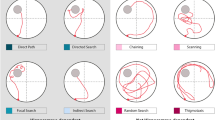
Alzheimer’s disease was modeled in female Wistar rats aged 4 months by stereotaxic bilateral injection of a synthetic peptide β-amyloid (Aβ1-42) into the hippocampus. Behavioral tests (open field, Y-maze, passive avoidance, and Morris water maze) revealed significant impairment of memory and spatial navigation 8 weeks after β-amyloid administration. At this term, the cognitive impairments typical of Alzheimer’s disease are reproduced. The experimental model of Alzheimer’s disease proposed by us can be used in preclinical studies of drugs for the treatment of this pathology.
Similar content being viewed by others
References
Solis E Jr, Hascup KN, Hascup ER. Alzheimer’s disease: The link between amyloid-β and neurovascular dysfunction. J. Alzheimers Dis. 2020;76(4):1179-1198. https://doi.org/10.3233/JAD-200473
Prince M, Albanese E, Guerchet M, Prina M. World Alzheimer Report 2014: Dementia and risk reduction an analysis of protective and modifiable factors. London: Alzheimer’s Disease International, 2014.
Barage SH, Sonawane KD. Amyloid cascade hypothesis: Pathogenesis and therapeutic strategies in Alzheimer’s disease. Neuropeptides. 2015;52:1-18. https://doi.org/10.1016/j.npep.2015.06.008
Lane CA, Hardy J, Schott JM. Alzheimer’s disease. Eur. J. Neurol. 2018;25(1):59-70. https://doi.org/10.1111/ene.13439
Khan S, Barve KH, Kumar MS. Recent advancements in pathogenesis, diagnostics and treatment of Alzheimer’s disease. Curr. Neuropharmacol. 2020;18(11):1106-1125. https://doi.org/10.2174/1570159X18666200528142429
Iptyshev AM, Gorina YaV, Lopatina OL, Komleva YuK, Salmina AB. Experimental models of Alzheimer’s disease: advantages and disadvantages. Sib. Med. Obozrenie. 2016;(4):5-21. Russian.
Drummond E, Wisniewski T. Alzheimer’s disease: experimental models and reality. Acta Neuropathol. 2017;133(2):155-175. https://doi.org/10.1007/s00401-016-1662-x
Baerends E, Soud K, Folke J, Pedersen AK, Henmar S, Konrad L, Lycas MD, Mori Y, Pakkenberg B, Woldbye DPD, Dmytriyeva O, Pankratova S. Modeling the early stages of Alzheimer’s disease by administering intracerebroventricular injections of human native Aβ oligomers to rats. Acta Neuropathol. Commun. 2022;10(1):113. https://doi.org/10.1186/s40478-022-01417-5
Petrasek T, Skurlova M, Maleninska K, Vojtechova I, Kristofikova Z, Matuskova H, Sirova J, Vales K, Ripova D, Stuchlik A. A rat model of Alzheimer’s disease based on a beta42 and pro-oxidative substances exhibits cognitive deficit and alterations in glutamatergic and cholinergic neurotransmitter systems. Front. Aging Neurosci. 2016;8:83. https://doi.org/10.3389/fnagi.2016.00083
Poon CH, Wang Y, Fung ML, Zhang C, Lim LW. Rodent models of amyloid-beta feature of Alzheimer’s disease: development and potential treatment implications. Aging Dis. 2020;11(5):1235-1259. https://doi.org/10.14336/AD.2019.1026
Mamun AA, Hashimoto M, Katakura M, Matsuzaki K, Hossain S, Arai H, Shido O. Neuroprotective effect of madecassoside evaluated using amyloid Β1-42-mediated in vitro and in vivo Alzheimer’s disease models. Int. J. Indigenous Med. Plants 2014;47(2):1669-1682.
Facchinetti R, Bronzuoli MR, Scuderi C. An animal model of Alzheimer disease based on the intrahippocampal injection of amyloid β-peptide (1-42). Methods Mol. Biol. 2018;1727:343-352. https://doi.org/10.1007/978-1-4939-7571-6_25
Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, 1998.
Morozova A, Zubkov E, Strekalova T, Kekelidze Z, Storozeva Z, Schroeter C.A, Bazhenova N, Lesch K.P, Cline B.H, Chekhonin V. Ultrasound of alternating frequencies and variable emotional impact evokes depressive syndrome in mice and rats. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;68):52-63. https://doi.org/10.1016/j.pnpbp. 2016.03.003
Zubkov EA, Zorkina YA, Gurina OI, Melnikov PA, Morozova AY, Chekhonin VP. Prenatal exposure to brain-specific anion transporter-1-specific monoclonal antibodies impairs cognitive function in post-natal life. Neuropeptides. 2017;65:100-105. https://doi.org/10.1016/j.npep.2017.07.001
Zhang HY, Zheng CY, Yan H, Wang ZF, Tang LL, Gao X, Tang XC. Potential therapeutic targets of huperzine A for Alzheimer’s disease and vascular dementia. Chem. Biol. Interact. 2008;175(1-3):396-402. https://doi.org/10.1016/j.cbi.2008.04.049
Winkler J, Connor DJ, Frautschy SA, Behl C, Waite JJ, Cole GM, Thal LJ. Lack of long-term effects after beta-amyloid protein injections in rat brain. Neurobiol. Aging. 1994;15(5):601-607. https://doi.org/10.1016/0197-4580(94)00054-9
O’Hare E, Scopes DI, Treherne JM, Monaghan J, Palmer PM, Amijee H, Kim EM. Novel anti-inflammatory compound SEN1176 alleviates behavioral deficits induced following bilateral intrahippocampal injection of aggregated amyloid-β1-42. J. Alzheimers Dis. 2011;25(2):219-229. https://doi.org/10.3233/JAD-2011-100044
McLarnon JG, Ryu JK. Relevance of a beta1-42 intrahippocampal injection as an animal model of inflamed Alzheimer’s disease brain. Curr. Alzheimer Res. 2008;5(5):475-480. https://doi.org/10.2174/156720508785908874
Perry G, Castellani RJ, Hirai K, Smith MA. Reactive oxygen species mediate cellular damage in Alzheimer disease. J. Alzheimers Dis. 1998;1(1):45-55. https://doi.org/10.3233/jad-1998-1103
Lin T, Liu GA, Perez E, Rainer RD, Febo M, Cruz-Almeida Y, Ebner NC. Systemic inflammation mediates age-related cognitive deficits. Front. Aging Neurosci. 2018;10:236. https://doi.org/10.3389/fnagi.2018.00236
McLarnon JG. Correlated inflammatory responses and neurodegeneration in peptide-injected animal models of Alzheimer’s disease. Biomed. Res. Int. 2014;2014:923670. https://doi.org/10.1155/2014/923670
Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 2008;14(8):837-842. https://doi.org/10.1038/nm1782
Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat. Neurosci. 2005;8(1):79-84. https://doi.org/10.1038/nn1372
Benedikz E, Kloskowska E, Winblad B. The rat as an animal model of Alzheimer’s disease. J. Cell. Mol. Med. 2009;13(6):1034-42. https://doi.org/10.1111/j.1582-4934.2009.00781.x
Paulo SL, Rodrigues RS, Shvachiy L, Ribeiro FF, Solá S, Sebastião AM, Xapelli S. Neurogenesis in a rat model of sporadic Alzheimer’s disease: PS227. Porto Biomed. J. 2017;2(5):205. https://doi.org/10.1016/j.pbj.2017.07.072
Zhu D, Yang N, Liu YY, Zheng J, Ji C, Zuo PP. M2 macrophage transplantation ameliorates cognitive dysfunction in amyloid-β-treated rats through regulation of microglial polarization. J. Alzheimers Dis. 2016;52(2):483-495. https://doi.org/10.3233/JAD-151090
Li S, Selkoe DJ. A mechanistic hypothesis for the impairment of synaptic plasticity by soluble Aβ oligomers from Alzheimer’s brain. J. Neurochem. 2020;154(6): 583-597. https://doi.org/10.1111/jnc.15007
Stepanova OV, Voronova AD, Chadin AV, Valikhov MP, Abakumov MA, Reshetov IV, Chekhonin VP. Isolation of rat olfactory ensheathing cells and their use in the therapy of posttraumatic cysts of the spinal cord. Bull. Exp. Biol. Med. 2018;165(1):132-135. https://doi.org/10.1007/s10517-018-4114-x
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Kletochnye Tekhnologii v Biologii i Meditsine, No. 1, pp. 14-19, March, 2023
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karsuntseva, E.K., Voronova, A.D., Chadin, A.V. et al. Application of Behavioral Tests for Evaluation of an Experimental Model of Alzheimer’s Disease in Female Rats. Bull Exp Biol Med 175, 126–131 (2023). https://doi.org/10.1007/s10517-023-05823-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-023-05823-0