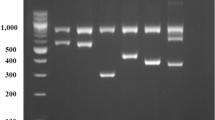
Drug acetylation plays an important role in the medical practice. Modern methods of acetylation phenotype prediction are based on genotyping of polymorphisms in the second exon of the gene NAT2. Some disadvantages of these methods limit their application in the clinical practice. We developed a method of human genotyping based on identification of NAT2 gene polymorphism rs1495741 by real-time PCR. This method of genotype determination has a number of advantages: high sensitivity, simplicity, possibility of automated interpretation of the results, and feasibility in clinical laboratories.
Similar content being viewed by others
References
Albom A, Norell S. Introduction to Modern Epidemiology. Tallin, 1996. Russian.
Gryadunov DA, Zimenkov DV, Mikhailovich VM, Nasedkina TV, Dementieva EI, Rubina AYu, Pan’kov SV, Barskikh VE, Zasedatelev AS. Hydrogel biochip technology and its application in clinical laboratory diagnostics. Med. Alfavit. 2009;1(2):58-59. Russian.
Ivashkin VT, Baranovsky AYu, Raikhelson KL, Palgova LK, Maevskaya MV, Kondrashina EA, Marchenko NV, Nekrasova TP, Nikitin IG. Drug-induced liver injuries (clinical guidelines for physicians). Russ. Zh. Gastroenterol., Hepatol., Koloproktol. 2019;29(1):101-131. Russian. https://doi.org/10.22416/1382-4376-2019-29-1-101-131
Mozhokina GN, Kazakov AV, Elistratova NA, Popov SA. Biotransformation enzymes for xenobiotics and personalization of treatment regimens for tuberculosis patients. Tuberk. Bolezni Legkikh. 2016;94(4):6-12. Russian. https://doi.org/10.21292/2075-1230-2016-94-4-6-12
Ogarkov OB, Peretolchina NP, Malov SI, Orlova EA, Stepanenko LA, Khromova PA. Patent RU No. 2756203. Method for determining human genotype associated with acetylation of xenobiotics. Bull. No. 28. Published September 28, 2021.
Chernyak YuI, Kolesnikov SI, Chernyak EV. Cytochrome P450: Main Conceptions, Methods of Research, Significance for Practical Medicine. Irkutsk, 2014. Russian.
Bolt HM, Selinski S, Dannappel D, Blaszkewicz M, Golka K. Re-investigation of the concordance of human NAT2 phenotypes and genotypes. Arch. Toxicol. 2005;79(4):196-200. https://doi.org/10.1007/s00204-004-0622-8
García-Closas M, Hein DW, Silverman D, Malats N, Yeager M, Jacobs K, Doll MA, Figueroa JD, Baris D, Schwenn M, Kogevinas M, Johnson A, Chatterjee N, Moore LE, Moeller T, Real FX, Chanock S, Rothman N. A single nucleotide polymorphism tags variation in the arylamine N-acetyltransferase 2 phenotype in populations of European background. Pharmacogenet. Genomics. 2011;21(4):231-236. https://doi.org/10.1097/FPC.0b013e32833e1b54
Hein DW. N-acetyltransferase SNPs: emerging concepts serve as a paradigm for understanding complexities of personalized medicine. Expert Opin. Drug Metab. Toxicol. 2009;5(4):353-366. https://doi.org/10.1517/17425250902877698
Hein DW, Doll MA, Rustan TD, Ferguson RJ. Metabolic activation of N-hydroxyarylamines and N-hydroxyarylamides by 16 recombinant human NAT2 allozymes: effects of 7 specific NAT2 nucleic acid substitutions. Cancer Res. 1995;55(16):3531-3536.
Ho HT, Wang TH, Hsiong CH, Perng WC, Wang NC, Huang TY, Jong YJ, Lu PL, Hu OY. The NAT2 tag SNP rs1495741 correlates with the susceptibility of antituberculosis drug-induced hepatotoxicity. Pharmacogenet. Genomics. 2013;23(4):200-207. https://doi.org/10.1097/FPC.0b013e32835e95e1
Kuznetsov IB, McDuffie M, Moslehi R. A web server for inferring the human N-acetyltransferase-2 (NAT2) enzymatic phenotype from NAT2 genotype. Bioinformatics. 2009;25(9):1185-1186. https://doi.org/10.1093/bioinformatics/btp121
McDonagh EM, Boukouvala S, Aklillu E, Hein DW, Altman RB, Klein TE. PharmGKB summary: very important pharmacogene information for N-acetyltransferase 2. Pharmacogenet. Genomics. 2014;24(8):409-425. https://doi.org/10.1097/FPC.0000000000000062
Zhang D, Hao J, Hou R, Yu Y, Hu B, Wei L. The role of NAT2 polymorphism and methylation in anti-tuberculosis drug-induced liver injury in Mongolian tuberculosis patients. J. Clin. Pharm. Ther. 2020;45(3):561-569. https://doi.org/10.1111/jcpt.13097
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Byulleten’ Eksperimental’noi Biologii i Meditsiny, Vol. 173, No. 4, pp. 527-531, April, 2022
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ogarkov, O.B., Peretolchina, N.P., Malov, S.I. et al. A Method to Determine Xenobiotic Acetylation Rate by Taq SNP rs1495741. Bull Exp Biol Med 173, 510–513 (2022). https://doi.org/10.1007/s10517-022-05572-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-022-05572-6