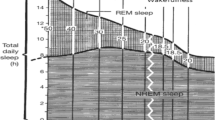
Analysis of the microstructure of sleep using extended EEG monitoring can provide deep understanding of the neuronal activity of the brain. Sleep spindles (SS) are one of the main EEG patterns occurring during the non-rapid eye movement sleep. SS reflect the process of synchronization and provide sleep initiation and maintenance by suppressing sensory information. SS are associated with a wide range of brain functions, such as memory and neuroplasticity, general intelligence and cognitive performance, which undergo various changes throughout the life. In this review we discuss the features of the formation and regression of SS in humans during ontogeny on the basis of published data of the last 5-6 years and fundamental results of previous studies at the Scientific Centre for Family Health and Human Reproduction Problems that formed the basis of the modern study of neurophysiological phenomena of the wakefulness and sleep. The search for diagnostic patterns and prognostic markers of the pathology of higher nervous activity remains a priority in fundamental studies and medical practice. Modern methods for studying sleep and its EEG patterns are the next step in understanding the neurophysiological aspects of the sleep—wake cycle. This will open prospects for predicting postnatal maturation, understanding the mechanisms of brain neuroplasticity in the “sleep—wakefulness” continuum, which is one of the tasks of modern somnology and neurophysiology.
Similar content being viewed by others
References
Berdina ON, Rychkova LV, Madaeva IM. Characteristics of sleep structure in schoolchildren with high intellectual abilities. Zh. Nevrol. Psikhiatr. 2018;118(7):78-81. Russian. doi: 10.17116/jnevro20181187178
Kolesnikov SI, Kolesnikova LI. Development of brain electrogenesis in children aged 1 to 7 years. Brain: Theoretical and Clinical Aspects. Pokrovsky VI, ed. Moscow, 2003. P. 193-241. Russian.
Korolyeva NV, Gutnik IN, Kolesnikov SI. Fundamentals of Clinical Electroencephalography. Irkutsk, 2005. Russian.
Korolyeva NV, Kolesnikov SI. Formation of Children’s Brain Bioelectric Activity in Ontogeny. Irkutsk, 2005. Russian.
Koroleva NV, Kolesnikov SI, Vorob’ev SV. Electroencephalographic Atlas of Epilepsies and Epileptic Syndromes in Children. Moscow, 2011. Russian.
Korolyova NV, Kolesnikov SI, Dolgih VV. Correlation between electroencephalographic parameters in children depending on the electroencephalogram type. Ultrazvuk. Funktsional. Diagnost. 2001;(2):122-132. Russian.
Koshchavtsev AG, Grechanyi SV. Interpretation of electroencephalography in infants. Epilepsiya Paroksizm. Sostoyaniya. 2020;12(1):9-25. Russian. doi: 10.17749/2077-8333.2020.12.1.9-25
Madaeva IM, Shevyrtalova ON, Dolgich VV. Arterial hypertension and sleep apnea in pediatrics: results of pilot trial. Pediatriya. Consilium Medicum. 2009;(3):114-116. Russian.
Melashenko TV, Guzeva VV. Particular transit patterns EEG in premature babies with hypoxia-ischemic encephalopathy. Pediatr. 2014;5(1):32-36. Russian.
Petrukhin AS, Sozaeva NS, Golosnaya GS. Neurobiologic and ontogenetic basis of motor functions development. Russ. Zh. Det. Nevrol. 2009;4(2):20-23. Russian.
Polyakov VM, Kolesnikova LI, Kolesnikov SI, Dolgikh VV, Kosovtseva AS, Rychkova LV, Prokhorova ZV. Peculiarities of functional hemispheric asymmetry formation in children and adolescents with hypertension. Vestn. Ross. Acad. Med. Nauk. 2014;69(9-10):77-82. Russian. doi: 10.15690/vramn.v69i9-10.1135
Shevyrtalova ON, Protopopova ON, Madayeva IM, Dolgikh VV, Kolesnikova LI, Polyakov VM, Prokhorova ZhV. Sleep disorders in the genesis of emotional personality and cognitive disorders in adolescents with essential hypertension. Ross. Pediatr. Zh. 2011;(2):12-16. Russian.
Aeschbach D, Borbély AA. All-night dynamics of the human sleep EEG. J. Sleep Res. 1993;2(2):70-81. doi: https://doi.org/10.1111/j.1365-2869.1993.tb00065.x
Ambrosius U, Lietzenmaier S, Wehrle R, Wichniak A, Kalus S, Winkelmann J, Bettecken T, Holsboer F, Yassouridis A, Friess E. Heritability of sleep electroencephalogram. Biol. Psychiatry. 2008;64(4):344-348. doi: https://doi.org/10.1016/j.biopsych.2008.03.002
Anderson CM, Torres F, Faoro A. The EEG of the early premature. Electroencephalogr. Clin. Neurophysiol. 1985;60(2):95-105. doi: https://doi.org/10.1016/0013-4694(85)90015-x
André M, Lamblin MD, d’Allest AM, Curzi-Dascalova L, Moussalli-Salefranque F, S Nguyen The T, Vecchierini-Blineau MF, Wallois F, Walls-Esquivel E, Plouin P. Electroencephalography in premature and full-term infants. Developmental features and glossary. Neurophysiol. Clin. 2010;40(2):59-124. doi: https://doi.org/10.1016/j.neucli.2010.02.002
Andrillon T, Nir Y, Staba RJ, Ferrarelli F, Cirelli C, Tononi G, Fried I. Sleep spindles in humans: insights from intracranial EEG and unit recordings. J. Neurosci. 2011;31(49):17 821-17 834. doi: 10.1523/JNEUROSCI.2604-11.2011
Antony JW, Schönauer M, Staresina BP, Cairney SA. Sleep spindles and memory reprocessing. Trends Neurosci. 2019;42(1):1-3. doi: https://doi.org/10.1016/j.tins.2018.09.012
Ayoub A, Aumann D, Hörschelmann A, Kouchekmanesch A, Paul P, Born J, Marshall L. Differential effects on fast and slow spindle activity, and the sleep slow oscillation in humans with carbamazepine and flunarizine to antagonize voltage-dependent Na+ and Ca2+ channel activity. Sleep. 2013;36(6):905-911. doi: https://doi.org/10.5665/sleep.2722
Berdina O, Madaeva I, Bolshakova S, Tsykunova M, Bugun O, Rychkova L. Applying a translated version of the adolescent sleep habits survey in Russian high school children with obesity. Int. J. Biomed. 2020;10(1):61-65. doi: https://doi.org/10.21103/Article10(1)_OA10
Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, Redline S, Strohl KP, Davidson Ward SL, Tangredi MM; American Academy of Sleep Medicine. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012;8(5):597-619. doi: https://doi.org/10.5664/jcsm.2172
Boylan G, Murray D, Rennie J. The normal EEG and aEEG. Neonatal Cerebral Investigation. Rennie J, Hagmann C, Robertson N, eds. Cambridge, 2008. P. 83-91. doi:https://doi.org/10.1017/CBO9780511544750.008
Campbell IG, Feinberg I. Maturational patterns of sigma frequency power across childhood and adolescence: a longitudinal study. Sleep. 2016;39(1):193-201. doi: https://doi.org/10.5665/sleep.5346
Cardozo PL, de Lima IBQ, Maciel EMA, Silva NC, Dobransky T, Ribeiro FM. Synaptic elimination in neurological disorders. Curr. Neuropharmacol. 2019;17(11):1071-1095. doi: https://doi.org/10.2174/1570159X17666190603170511
Clawson BC, Durkin J, Aton SJ. Form and function of sleep spindles across the lifespan. Neural. Plast. 2016;2016:6936381. doi: https://doi.org/10.1155/2016/6936381
Counsell SJ, Maalouf EF, Fletcher AM, Duggan P, Battin M, Lewis HJ, Herlihy AH, Edwards AD, Bydder GM, Rutherford MA. MR imaging assessment of myelination in the very preterm brain. AJNR Am. J. Neuroradiol. 2002;23(5):872-881.
Cox R, Schapiro AC, Manoach DS, Stickgold R. Individual differences in frequency and topography of slow and fast sleep spindles. Front. Hum. Neurosci. 2017;11:433. doi: https://doi.org/10.3389/fnhum.2017.00433
Crowley K, Trinder J, Kim Y, Carrington M, Colrain IM. The effects of normal aging on sleep spindle and K-complex production. Clin. Neurophysiol. 2002;113(10)1615-1622. doi: https://doi.org/10.1016/s1388-2457(02)00237-7
D’Atri A, Novelli L, Ferrara M, Bruni O, De Gennaro L. Different maturational changes of fast and slow sleep spindles in the first four years of life. Sleep Med. 2018;42:73-82. doi: https://doi.org/10.1016/j.sleep.2017.11.1138
De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med. Rev. 2003;7(5):423-440. doi: https://doi.org/10.1053/smrv.2002.0252
Destexhe A, Contreras D, Steriade M. Cortically-induced coherence of a thalamic-generated oscillation. Neuroscience. 1999;92(2):427-443. doi: https://doi.org/10.1016/s0306-4522(99)00024-x
Ellingson RJ. Development of sleep spindle bursts during the first year of life. Sleep. 1982;5(1):39-46.
Ellingson RJ, Peters JF. Development of EEG and daytime sleep patterns in Trisomy-21 infants during the first year of life: longitudinal observations. Electroencephalogr. Clin. Neurophysiol. 1980;50(5-6):457-466. doi: https://doi.org/10.1016/0013-4694(80)90012-7
Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J. Psychiatr. Res. 1982-1983;17(4):319-334. doi: https://doi.org/10.1016/0022-3956(82)90038-3
Feinberg I, Campbell IG. Sleep EEG changes during adolescence: an index of a fundamental brain reorganization. Brain Cogn. 2010;72(1):56-65. doi: https://doi.org/10.1016/j.bandc.2009.09.008
Feinberg I, Campbell IG. Longitudinal sleep EEG trajectories indicate complex patterns of adolescent brain maturation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;304(4):R296-R303. doi: https://doi.org/10.1152/ajpregu.00422.2012
Fernandez LMJ, Lüthi A. Sleep spindles: mechanisms and functions. Physiol. Rev. 2020;100(2):805-868. doi: https://doi.org/10.1152/physrev.00042.2018
Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Walhovd KB. High consistency of regional cortical thinning in aging across multiple samples. Cereb. Cortex. 2009;19(9):2001-2012. doi: https://doi.org/10.1093/cercor/bhn232
Goldstone A, Willoughby AR, de Zambotti M, Clark DB, Sullivan EV, Hasler BP, Franzen PL, Prouty DE, Colrain IM, Baker FC. Sleep spindle characteristics in adolescents. Clin. Neurophysiol. 2019;130(6):893-902. doi: https://doi.org/10.1016/j.clinph.2019.02.019
Goldstone A, Willoughby AR, de Zambotti M, Franzen PL, Kwon D, Pohl KM, Pfefferbaum A, Sullivan EV, Müller-Oehring EM, Prouty DE, Hasler BP, Clark DB, Colrain IM, Baker FC. The mediating role of cortical thickness and gray matter volume on sleep slow-wave activity during adolescence. Brain Struct. Funct. 2018;223(2):669-685. doi: https://doi.org/10.1007/s00429-017-1509-9
Gorgoni M, Lauri G, Truglia I, Cordone S, Sarasso S, Scarpelli S, Mangiaruga A, D’Atri A, Tempesta D, Ferrara M, Marra C, Rossini PM, De Gennaro L. Parietal fast sleep spindle density decrease in Alzheimer’s disease and amnesic mild cognitive impairment. Neural Plast. 2016;2016:8376108. doi: https://doi.org/10.1155/2016/8376108
Gruber R, Wise MS, Frenette S, Knäauper B, Boom A, Fontil L, Carrier J. The association between sleep spindles and IQ in healthy school-age children. Int. J. Psychophysiol. 2013;89(2):229-240. doi: https://doi.org/10.1016/j.ijpsycho.2013.03.018
Gunn DG, Naismith SL, Terpening Z, Lewis SJ. The relationships between poor sleep efficiency and mild cognitive impairment in Parkinson disease. J. Geriatr. Psychiatry Neurol. 2014;27(2):77-84. doi: https://doi.org/10.1177/0891988713509135
Hagne I. Development of the EEG in normal infants during the first year of life. A longitudinal study. Acta Paediatr. Scand. Suppl. 1972;232:1-53.
Hata Y. Synaptic Elimination. Encyclopedia of Neuroscience. Binder MD, Hirokawa N, Windhorst U, eds. Berlin, 2009.
Holz J, Piosczyk H, Feige B, Spiegelhalder K, Baglioni C, Riemann D, Nissen C. EEG Σ and slow-wave activity during NREM sleep correlate with overnight declarative and procedural memory consolidation. J. Sleep Res. 2012;21(6):612-619. doi: https://doi.org/10.1111/j.1365-2869.2012.01017.x
Jankel WR, Niedermeyer E. Sleep spindles. J. Clin. Neurophysiol. 1985;2(1):1-35. doi: https://doi.org/10.1097/00004691-198501000-00001
Kaminska A, Eisermann M, Plouin P. Child EEG (and maturation). Handb. Clin. Neurol. 2019;160:125-142. doi: https://doi.org/10.1016/B978-0-444-64032-1.00008-4
Khazipov R, Luhmann HJ. Early patterns of electrical activity in the developing cerebral cortex of humans and rodents. Trends Neurosci. 2006;29(7):414-418. doi: https://doi.org/10.1016/j.tins.2006.05.007
Knickmeyer RC, Gouttard S, Kang C, Evans D, Wilber K, Smith JK, Hamer RM, Lin W, Gerig G, Gilmore JH. A structural MRI study of human brain development from birth to 2 years. J. Neurosci. 2008;28(47):12 176-12 182. doi: 10.1523/JNEUROSCI.3479-08.2008
Koszer SE, Moshé SL, Holmes GL. Visual analysis of the neonatal electroencephalogram. Clinical Neurophysiology of Infancy, Childhood, and Adolescence. Elsevier, 2006. P. 70-86.
Landolt HP. Genetic determination of sleep EEG profiles in healthy humans. Prog. Brain Res. 2011;193:51-61. doi: https://doi.org/10.1016/B978-0-444-53839-0.00004-1
Latreille V, Carrier J, Lafortune M, Postuma RB, Bertrand JA, Panisset M, Chouinard S, Gagnon JF. Sleep spindles in Parkinson’s disease may predict the development of dementia. Neurobiol. Aging. 2015;36(2):1083-1090. doi: https://doi.org/10.1016/j.neurobiolaging.2014.09.009
Leger D, Beck F, Richard JB, Godeau E. Total sleep time severely drops during adolescence. PLoS One. 2012;7(10):e45204. doi: https://doi.org/10.1371/journal.pone.0045204
Liu S, Pan J, Tang K, Lei Q, He L, Meng Y, Cai X, Li Z. Sleep spindles, K-complexes, limb movements and sleep stage proportions may be biomarkers for amnestic mild cognitive impairment and Alzheimer’s disease. Sleep Breath. 2020;24(2):637-651. doi: https://doi.org/10.1007/s11325-019-01970-9
Loomis AL, Harvey EN, Hobart G. Potential rhythms of the cerebral cortex during sleep. Science. 1935;81:597-598. doi: https://doi.org/10.1126/science.81.2111.597
Lüthi A. Sleep spindles: where they come from, what they do. Neuroscientist. 2014;20(3):243-256. doi: https://doi.org/10.1177/1073858413500854
Madaeva I, Shevyrtalova O, Dolgikh V, Kolesnikova L. Obstructive sleep apnea/hypopnea syndrome in adolescents with essential hypertension. Sleep Med. 2009;10(10):1167-1168. doi: https://doi.org/10.1016/j.sleep.2009.04.002
Mak-McCully RA, Rolland M, Sargsyan A, Gonzalez C, Magnin M, Chauvel P, Rey M, Bastuji H, Halgren E. Coordination of cortical and thalamic activity during non-REM sleep in humans. Nat. Commun. 2017;8:15499. doi: https://doi.org/10.1038/ncomms15499
Mander BA, Winer JR, Walker MP. Sleep and human aging. Neuron. 2017;94(1):19-36. doi: https://doi.org/10.1016/j.neuron.2017.02.004
Martin N, Lafortune M, Godbout J, Barakat M, Robillard R, Poirier G, Bastien C, Carrier J. Topography of age-related changes in sleep spindles. Neurobiol. Aging. 2013;34(2):468-476. doi: https://doi.org/10.1016/j.neurobiolaging.2012.05.020
Milh M, Kaminska A, Huon C, Lapillonne A, Ben-Ari Y, Khazipov R. Rapid cortical oscillations and early motor activity in premature human neonate. Cereb. Cortex. 2007;17(7):1582-1594. doi: https://doi.org/10.1093/cercor/bhl069
Nelson PT, Abner EL, Scheff SW, Schmitt FA, Kryscio RJ, Jicha GA, Smith CD, Patel E, Markesbery WR. Alzheimer’s-type neuropathology in the precuneus is not increased relative to other areas of neocortex across a range of cognitive impairment. Neurosci. Lett. 2009;450(3):336-339. doi: https://doi.org/10.1016/j.neulet.2008.11.006
Nicolas A, Petit D, Rompré S, Montplaisir J. Sleep spindle characteristics in healthy subjects of different age groups. Clin. Neurophysiol. 2001;112(3):521-527. doi: https://doi.org/10.1016/s1388-2457(00)00556-3
Northcutt RG. Body and Brain. A Trophic Theory of Neural Connections. Dale Purves. Harvard University Press, Cambridge, MA, 1988. viii, 231 pp., illus. $35. Science. 1989;244:993. doi: 10.1126/science.244.4907.993
Peter-Derex L, Comte JC, Mauguière F, Salin PA. Density and frequency caudo-rostral gradients of sleep spindles recorded in the human cortex. Sleep. 2012;35(1):69-79. doi: https://doi.org/10.5665/sleep.1588
Picard-Deland C, Carr M, Paquette T, Saint-Onge K, Nielsen T. Sleep spindle and psychopathology characteristics of frequent nightmare recallers. Sleep Med. 2018;50:113-131. doi: https://doi.org/10.1016/j.sleep.2017.10.003
Purcell SM, Manoach DS, Demanuele C, Cade BE, Mariani S, Cox R, Panagiotaropoulou G, Saxena R, Pan JQ, Smoller JW, Redline S, Stickgold R. Characterizing sleep spindles in 11,630 individuals from the National Sleep Research Resource. Nat. Commun. 2017;8:15930. doi: https://doi.org/10.1038/ncomms15930
Rauchs G, Schabus M, Parapatics S, Bertran F, Clochon P, Hot P, Denise P, Desgranges B, Eustache F, Gruber G, Anderer P. Is there a link between sleep changes and memory in Alzheimer’s disease? Neuroreport. 2008;19(11):1159-1162. doi: https://doi.org/10.1097/WNR.0b013e32830867c4
Reichert CF, Veitz S, Bühler M, Gruber G, Deuring G, Rehm SS, Rentsch K, Garbazza C, Meyer M, Slawik H, Lin YS, Weibel J. Wide awake at bedtime? Effects of caffeine on sleep and circadian timing in male adolescents — a randomized crossover trial. Biochem. Pharmacol. 2021;191:114283. doi: https://doi.org/10.1016/j.bcp.2020.114283
Reynolds CM, Gradisar M, Coussens S, Short MA. Sleep spindles in adolescence: a comparison across sleep restriction and sleep extension. Sleep Med. 2018;50:166-174. doi: https://doi.org/10.1016/j.sleep.2018.05.019
Rychkova L, Madaeva I, Berdina O, Bolshakova S, Bugun O. 211 Inadequate sleep habits ARE associated with obesity in high school children. Arch. Dis. Childhood. 2021;106:A89. doi: https://doi.org/10.1136/archdischild-2021-europaediatrics.211
Schabus M, Dang-Vu TT, Albouy G, Balteau E, Boly M, Carrier J, Darsaud A, Degueldre C, Desseilles M, Gais S, Phillips C, Rauchs G, Schnakers C, Sterpenich V, Vandewalle G, Luxen A, Maquet P. Hemodynamic cerebral correlates of sleep spindles during human non-rapid eye movement sleep. Proc. Natl Acad. Sci. USA. 2007;104(32):13164-13169. doi: https://doi.org/10.1073/pnas.0703084104
Scholle S, Zwacka G, Scholle HC. Sleep spindle evolution from infancy to adolescence. Clin. Neurophysiol. 2007;118(7):1525-1531. doi: https://doi.org/10.1016/j.clinph.2007.03.007
Seibt J, Timofeev I, Carrier J, Peyrache A. Role of spindle oscillations across lifespan in health and disease. Neural Plast. 2016;2016:8103439. doi: https://doi.org/10.1155/2016/8103439
Shinomiya S, Nagata K, Takahashi K, Masumura T. Development of sleep spindles in young children and adolescents. Clin. Electroencephalogr. 1999;30(2):39-43. doi: https://doi.org/10.1177/155005949903000203
Skeldon AC, Derks G, Dijk DJ. Modelling changes in sleep timing and duration across the lifespan: Changes in circadian rhythmicity or sleep homeostasis? Sleep Med. Rev. 2016;28:96-107. doi: https://doi.org/10.1016/j.smrv.2015.05.011
Steriade M, Timofeev I. Neuronal plasticity in thalamocortical networks during sleep and waking oscillations. Neuron. 2003;37(4):563-576. doi: https://doi.org/10.1016/s0896-6273(03)00065-5
Tarokh L, Carskadon MA. Developmental changes in the human sleep EEG during early adolescence. Sleep. 2010;33(6):801-809. doi: https://doi.org/10.1093/sleep/33.6.801
Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology. 2010;35(1):147-168. doi: https://doi.org/10.1038/npp.2009.115
Tsuchida TN, Wusthoff CJ, Shellhaas RA, Abend NS, Hahn CD, Sullivan JE, Nguyen S, Weinstein S, Scher MS, Riviello JJ, Clancy RR; American Clinical Neurophysiology Society Critical Care Monitoring Committee. American clinical neurophysiology society standardized EEG terminology and categorization for the description of continuous EEG monitoring in neonates: report of the American Clinical Neurophysiology Society critical care monitoring committee. J. Clin. Neurophysiol. 2013;30(2):161-173. doi: https://doi.org/10.1097/WNP.0b013e3182872b24
Volpe JJ. Specialized studies in the neurological evaluation. Neurology of the Newborn. Philadelphia, 2008. P. 154-202.
Warby SC, Wendt SL, Welinder P, Munk EG, Carrillo O, Sorensen HB, Jennum P, Peppard PE, Perona P, Mignot E. Sleep-spindle detection: crowdsourcing and evaluating performance of experts, non-experts and automated methods. Nat. Methods. 2014;11(4):385-392. doi: https://doi.org/10.1038/nmeth.2855
Weaver E, Gradisar M, Dohnt H, Lovato N, Douglas P. The effect of presleep video-game playing on adolescent sleep. J. Clin. Sleep Med. 2010;6(2):184-189.
Westlye LT, Walhovd KB, Dale AM, Bjørnerud A, Due-Tønnessen P, Engvig A, Grydeland H, Tamnes CK, Østby Y, Fjell AM. Differentiating maturational and aging-related changes of the cerebral cortex by use of thickness and signal intensity. Neuroimage. 2010;52(1):172-185. doi: https://doi.org/10.1016/j.neuroimage.2010.03.056
Whitehead K, Pressler R, Fabrizi L. Characteristics and clinical significance of delta brushes in the EEG of premature infants. Clin. Neurophysiol. Pract. 2016;2:12-18. doi: https://doi.org/10.1016/j.cnp.2016.11.002
Whitelaw BS. Microglia-mediated synaptic elimination in neuronal development and disease. J. Neurophysiol. 2018;119(1):1-4. doi: https://doi.org/10.1152/jn.00021.2017
Zhang ZY, Campbell IG, Dhayagude P, Espino HC, Feinberg I. Longitudinal analysis of sleep spindle maturation from childhood through late adolescence. J. Neurosci. 2021;41(19):4253-4261. doi: https://doi.org/10.1523/JNEUROSCI.2370-20.2021
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Byulleten’ Eksperimental’noi Biologii i Meditsiny, Vol. 173, No. 4, pp. 404-415, April, 2022
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ukhinov, E.B., Madaeva, I.M., Berdina, O.N. et al. Features of the EEG Pattern of Sleep Spindles and Its Diagnostic Significance in Ontogeny. Bull Exp Biol Med 173, 399–408 (2022). https://doi.org/10.1007/s10517-022-05557-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-022-05557-5