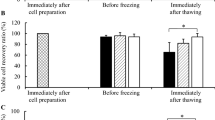
We studied the influence of sucrose applied in combination with different concentrations of penetrating cryoprotectants (DMSO, ethylene glycol, and glycerol) on the efficiency of cryopreservation of umbilical cord-derived multipotent stromal cells (MSC). The results indicate that these cells can be cryopreserved with the use of 5-10% DMSO or ethylene glycol with equal efficiency; addition of 0.2 M sucrose does not affect cell survival after thawing. The efficiency of glycerol as a cryoprotectant increases with increasing its concentration from 5 to 10%, but remains significantly lower than the efficiency of DMSO or ethylene glycol. Addition of sucrose to a final concentration of 0.2 M increases the efficiency of glycerol. The efficiency of combination of 10% glycerol and sucrose was comparable with that of combinations of DMSO and ethylene glycol with sucrose. The mechanism of the observed enhancement is apparently related to the influence of sucrose on the dynamic properties of the lipid membranes and facilitation of glycerol diffusion into the cells.
Similar content being viewed by others
References
Arutyunyan I, Elchaninov A, Makarov A, Fatkhudinov T. Umbilical cord as prospective source for mesenchymal stem cell-based therapy. Stem Cells Int. 2016;2016:6901286. doi: https://doi.org/10.1155/2016/6901286
Arutyunyan I, Fatkhudinov T, Sukhikh G. Umbilical cord tissue cryopreservation: a short review. Stem Cell Res. Ther. 2018;9(1):236. doi: https://doi.org/10.1186/s13287-018-0992-0
Arutyunyan IV, Strokova SО, Makarov АV, Mullabaeva SM, Elchaninov АV, Lokhonina АV, Abramov АА, Fatkhudinov ТK. DMSO-free cryopreservation of human umbilical cord tissue. Bull. Exp. Biol. Med. 2018;166(1):155-162. doi: https://doi.org/10.1007/s10517-018-4305-5
Best BP. Cryoprotectant toxicity: facts, issues, and questions. Rejuvenation Res. 2015;18(5):422-436. doi: https://doi.org/10.1089/rej.2014.1656
de Lima Prata K, de Santis GC, Orellana MD, Palma PV, Brassesco MS, Covas DT. Cryopreservation of umbilical cord mesenchymal cells in xenofree conditions. Cytotherapy. 2012;14(6):694-700. doi: https://doi.org/10.3109/14653249.2012.677820
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317. doi: https://doi.org/10.1080/14653240600855905
Duan W, Lopez MJ, Hicok K. Adult multipotent stromal cell cryopreservation: Pluses and pitfalls. Vet. Surg. 2018;47(1):19-29. doi: https://doi.org/10.1111/vsu.12730
Elliott GD, Wang S, Fuller BJ. Cryoprotectants: A review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology. 2017;76:74-91. doi: https://doi.org/10.1016/j.cryobiol.2017.04.004
Fuller BJ. Cryoprotectants: the essential antifreezes to protect life in the frozen state. Cryo Letters. 2004;25(6):375-388.
Gurruchaga H, Saenz Del Burgo L, Garate A, Delgado D, Sanchez P, Orive G, Ciriza J, Sanchez M, Pedraz JL. Cryopreservation of human mesenchymal stem cells in an allogeneic bioscaffold based on platelet rich plasma and synovial fluid. Sci. Rep. 2017;7(1):15733. doi: https://doi.org/10.1038/s41598-017-16134-6
Hendijani F. Explant culture: An advantageous method for isolation of mesenchymal stem cells from human tissues. Cell Prolif. 2017;50(2):e12334. doi: https://doi.org/10.1111/cpr.12334
Kalaszczynska I, Ferdyn K. Wharton’s jelly derived mesenchymal stem cells: future of regenerative medicine? Recent findings and clinical significance. Biomed. Res. Int. 2015;2015:430847. doi: https://doi.org/10.1155/2015/430847
Leonov DV, Dzuba SA, Surovtsev NV. Membrane-sugar interactions probed by low-frequency raman spectroscopy: the monolayer adsorption model. Langmuir. 2020;36(39):11655-11660. doi: https://doi.org/10.1021/acs.langmuir.0c02458
Li YH, Cai KJ, Kovacs A, Ji WZ. Effects of various extenders and permeating cryoprotectants on cryopreservation of cynomolgus monkey (Macaca fascicularis) spermatozoa. J. Androl. 2005;26(3):387-395. doi: https://doi.org/10.2164/jandrol.04147
Malo C, Crichton EG, Skidmore JA. Optimization of the cryopreservation of dromedary camel semen: Cryoprotectants and their concentration and equilibration times. Cryobiology. 2017;74:141-147. doi: https://doi.org/10.1016/j.cryobiol.2016.11.001
Mantri S, Kanungo S, Mohapatra PC. Cryoprotective effect of disaccharides on cord blood stem cells with minimal use of DMSO. Indian J. Hematol. Blood Transfus. 2015;31(2):206-212. doi: https://doi.org/10.1007/s12288-014-0352-x
Marquez-Curtis LA, Janowska-Wieczorek A, McGann LE, Elliott JA. Mesenchymal stromal cells derived from various tissues: Biological, clinical and cryopreservation aspects. Cryobiology. 2015;71(2):181-197. doi: https://doi.org/10.1016/j.cryobiol.2015.07.003
Rogulska O, Petrenko Y, Petrenko A. DMSO-free cryopreservation of adipose-derived mesenchymal stromal cells: expansion medium affects post-thaw survival. Cytotechnology. 2017;69(2):265-276. doi: https://doi.org/10.1007/s10616-016-0055-2
Rogulska O, Tykhvynska O, Revenko O, Grischuk V, Mazur S, Volkova N, Vasyliev R, Petrenko A, Petrenko Y. Novel cryopreservation approach providing off-the-shelf availability of human multipotent mesenchymal stromal cells for clinical applications. Stem Cells Int. 2019;2019:4150690. doi: https://doi.org/10.1155/2019/4150690
Roy S, Arora S, Kumari P, Ta M. A simple and serum-free protocol for cryopreservation of human umbilical cord as source of Wharton’s jelly mesenchymal stem cells. Cryobiology. 2014;68(3):467-472. doi: https://doi.org/10.1016/j.cryobiol.2014.03.010
Santiani A, Evangelista-Vargas S, Vargas S, Gallo S, Ruiz L, Orozco V, Rosemberg M. Cryopreservation of Peruvian Paso horse spermatozoa: dimethylacetamide preserved an optimal sperm function compared to dimethyl sulfoxide, ethylene glycol and glycerol. Andrologia. 2017;49(6). doi: 10.1111/and.12672
Shah SA, Andrabi SM, Qureshi IZ. Effect of equilibration times, freezing, and thawing rates on post-thaw quality of buffalo (Bubalus bubalis) bull spermatozoa. Andrology. 2016;4(5):972-976. doi: https://doi.org/10.1111/andr.12214
Shahverdi A, Rastegarnia A, Rezaei Topraggaleh T. Effect of extender and equilibration time on post thaw motility and chromatin structure of buffalo bull (bubalus bubalis) spermatozoa. Cell J. 2014;16(3):279-288.
Shivakumar SB, Bharti D, Subbarao RB, Jang SJ, Park JS, Ullah I, Park JK, Byun JH, Park BW, Rho GJ. DMSO- and serumfree cryopreservation of Wharton’s jelly tissue isolated from human umbilical cord. J. Cell. Biochem. 2016;117(10):2397-2412. doi: https://doi.org/10.1002/jcb.25563
Svoradová A, Kuželová L, Vašíček J, Baláži A, Hanusová E, Chrenek P. In vitro effect of various cryoprotectants on the semen quality of endangered Oravka chicken. Zygote. 2018;26(1):33-39. doi: https://doi.org/10.1017/S0967199417000685
Vian AM, Higgins AZ. Membrane permeability of the human granulocyte to water, dimethyl sulfoxide, glycerol, propylene glycol and ethylene glycol. Cryobiology. 2014;68(1):35-42. doi: https://doi.org/10.1016/j.cryobiol.2013.11.004
Woods EJ, Perry BC, Hockema JJ, Larson L, Zhou D, Goebel WS. Optimized cryopreservation method for human dental pulp-derived stem cells and their tissues of origin for banking and clinical use. Cryobiology. 2009;59(2):150-157. doi: https://doi.org/10.1016/j.cryobiol.2009.06.005
Yang Y, Melzer C, Bucan V, von der Ohe J, Otte A, Hass R. Conditioned umbilical cord tissue provides a natural threedimensional storage compartment as in vitro stem cell niche for human mesenchymal stroma/stem cells. Stem Cell Res. Ther. 2016;7:28. doi: https://doi.org/10.1186/s13287-016-0289-0
Yong KW, Choi JR, Wan Safwani WK. Biobanking of human mesenchymal stem cells: future strategy to facilitate clinical applications. Adv. Exp. Med. Biol. 2016;951:99-110. doi: https://doi.org/10.1007/978-3-319-45457-3_8
Zhang TY, Tan PC, Xie Y, Zhang XJ, Zhang PQ, Gao YM, Zhou SB, Li QF. The combination of trehalose and glycerol: an effective and non-toxic recipe for cryopreservation of human adipose-derived stem cells. Stem Cell Res. Ther. 2020;11(1):460. doi: https://doi.org/10.1186/s13287-020-01969-0
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Kletochnye Tekhnologii v Biologii i Meditsine, No. 1, pp. 47-53, March, 2021
Rights and permissions
About this article
Cite this article
Arutyunyan, I.V., Kananykhina, E.Y., Elchaninov, A.V. et al. Influence of Sucrose on the Efficiency of Cryopreservation of Human Umbilical Cord-Derived Multipotent Stromal Cells with the Use of Various Penetrating Cryoprotectants. Bull Exp Biol Med 171, 150–155 (2021). https://doi.org/10.1007/s10517-021-05187-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-021-05187-3