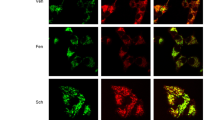
We have previously demonstrated that the development of oxidative stress in some pathologies can be prevented by activation of the mitochondrial ATP-dependent potassium channel (mitoKATP). Here we studied the effect of modulation of mitoKATP on the development of mitochondrial and endothelial dysfunction in the medulla oblongata and myocardium of rats with experimental parkinsonism. It is known that uridine-5’-diphosphate, activator of mitoKATP, does not penetrate the plasma membrane, but it can be synthesized in cells from exogenous uridine that is delivered into cells by special transport systems. Our results suggest that mitoKATP is involved in the development of mitochondrial and endothelial dysfunction in experimental parkinsonism and prove the cardio- and neuroprotective effects of uridine.
Similar content being viewed by others
References
Krylova IB, Bulion VV, Selina EN, Mironova GD, Sapronov NS. Effect of uridine on energy metabolism, LPO, and antioxidant system in the myocardium under conditions of acute coronary insufficiency. Bull. Exp. Biol. Med. 2012;153(5):644-646. https://doi.org/10.1007/s10517-012-1787-4
Mab’kovksaya IN, Gonchar OA, Nosar’ VI, Rozova EV, Bratus’ LV, Kolesnikova EE, Putii YuV, Karaban IN. Mitochondrial dysfunction and oxidative disturbances in the brain of rats with simulated Parkinsonian syndrome: the corrective effect of capicor. Fiziol. Zh. 2018;64(4):82-90.
Frelikh GA, Polomeeva NU, Vasil’ev AS, Udut VV. State-ofthe art methods of evaluation of mitochondrial function. Sib. Med. Zh. 2013;28(3):7-13. Russian.
Burbulla LF, Song P, Mazzulli JR, Zampese E, Wong YC, Jeon S, Santos DP, Blanz J, Obermaier CD, Strojny C, Savas JN, Kiskinis E, Zhuang X, Krüger R, Surmeier DJ, Krainc D. Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Science. 2017;357:1255-1261. https://doi.org/10.1126/science.aam9080
Campello S, Scorrano L. Mitochondrial shape changes: orchestrating cell pathophysiology. EMBO Rep. 2010;11(9):678-684. https://doi.org/10.1038/embor.2010.115
Cogliati S, Frezza C, Soriano ME, Varanita T, Quintana-Cabrera R, Corrado M, Cipolat S, Costa V, Casarin A, Gomes LC, Perales-Clemente E, Salviati L, Fernandez-Silva P, Enriquez J.A, Scorrano L. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell. 2013;155(1):160-171. https://doi.org/10.1016/j.cell.2013.08.032
Gao J, Wang L, Liu J, Xie F, Su B, Wang X. Abnormalities of Mitochondrial Dynamics in Neurodegenerative Diseases. Antioxidants (Basel). 2017;6(2):25. https://doi.org/10.3390/antiox6020025
Gonchar O, Mankovska I, Rozova K, Bratus L, Karaban I. Nonel approaches to correction of mitochondrial dysfunction and oxidative disorders in Parkinson’s disease. Fiziol. Zh. 2019;65(3):61-72.
Karaban I, Mankovska I, Rozova K, Bratus L, Gonchar O. Pharmacological targeting of mitochondrial dysfunction in Parkinson’s disease: approaches and perspectives. Drug Target Rev. 2018;(3):49-53.
Mironova GD, Khrenov MO, Talanov EYu, Glushkova OV, Parfenyuk SB, Novoselova TV, Lunin SM, Belosludtseva NV, Novoselova EG, Lemasters JJ. The role of mitochondrial KATP channel in anti-inflammatory effects of uridine in endotoxemic mice. Arch. Biochem. Biophys. 2018;654:70-76. https://doi.org/10.1016/j.abb.2018.07.006
Mironova GD, Negoda AE, Marinov BS, Paucek P, Costa ADT, Grigoriev SM, Skarga YYu, Garlid KD. Functional distinctions between the mitochondrial ATP-dependent K+ channel (mitoKATP) and its inward rectifier subunit (mitoKIR). J. Biol. Chem. 2004;279(31):32 562-32 568. https://doi.org/10.1074/jbc.M401115200
Mironova GD, Rozova EV, Belosludtseva NV, Man’kovskaya IN. Dynamic Restructuring of the Myocardial Mitochondria in Response to Uridine Modulation of the Activity of Mitochondrial ATP-Dependent Potassium Channel under Conditions of Acute Hypoxic Hypoxia. Bull. Exp. Biol. Med. 2019;166(6):806-810. https://doi.org/10.1007/s10517-019-04445-9
Mironova GD, Shigaeva MI, Gritsenko EN, Murzaeva SV, Gorbacheva OS, Germanova EL, Lukyanova LD. Functioning of the mitochondrial ATP-dependent potassium channel in rats varying in their resistance to hypoxia. Involvement of the channel in the process of animal’s adaptation to hypoxia. J. Bioenerg. Biomembr. 2010;42(6):473-481. https://doi.org/10.1007/s10863-010-9316-5
Rozova EV, Mankovskaya IN, Belosludtseva NV, Khmil NV, Mironova GD. Uridine as a protector against hypoxia-induced lung injury. Sci. Rep. 2019;9(1):9418. https://doi.org/10.1038/s41598-019-45979-2
Zhang Y, Guo S, Xie C, Fang J. Uridine Metabolism and Its Role in Glucose, Lipid, and Amino Acid Homeostasis. Biomed. Res. Int. 2020;2020:7091718. https://doi.org/10.1155/2020/7091718
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Byulleten’ Eksperimental’noi Biologii i Meditsiny, Vol. 170, No. 10, pp. 437-442, October, 2020
Rights and permissions
About this article
Cite this article
Mosentsov, A.A., Rozova, E.V., Belosludtseva, N.V. et al. Does the Operation of Mitochondrial ATP-Dependent Potassium Channels Affect the Structural Component of Mitochondrial and Endothelial Dysfunctions in Experimental Parkinsonism?. Bull Exp Biol Med 170, 431–435 (2021). https://doi.org/10.1007/s10517-021-05081-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-021-05081-y