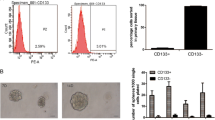
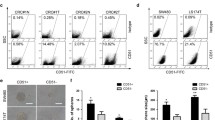
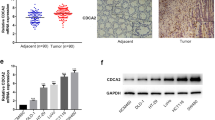
We studied proliferative activity of colorectal cancer cells with different expression level of CD133 molecule associated with cancer stem cells phenotype. Analysis of BrdU incorporation into Caco-2 and HT-29 cell lines showed that the percentage of cells in the DNA synthesis phase in the CD133+/high population is higher than in CD133—/low population. The expression of proliferation marker Ki-67 and the percentage of Ki-67+ cells were also higher in the CD133+/high population. Colorimetric analysis with crystal violet dye showed that the number of cells after 10-days culturing was higher in the CD133+/high population in both cell lines. These findings suggest that cells with high level of CD133 expression are characterized by higher proliferative activity, which can contribute to the tumor progression.
Similar content being viewed by others
References
Suvorov RE, Kim YS, Gisina AM, Chiang JH, Yarygin KN, Lupatov AY. Surface Molecular Markers of Cancer Stem Cells: Computation Analysis of Full-Text Scientific Articles. Bull. Exp. Biol. Med. 2018;166(1):135-140.
Batlle E, Clevers H. Cancer stem cells revisited. Nat. Med.2017; 23(10):1124-1134.
Chang PH, Sekine K, Chao HM, Hsu SH, Chern E. Chitosan promotes cancer progression and stem cell properties in association with Wnt signaling in colon and hepatocellular carcinoma cells. Sci. Rep. 2017;8. ID 45751. doi: https://doi.org/10.1038/srep45751
Corbeil D, Röper K, Fargeas C.A, Joester A, Huttner W.B. Prominin: a story of cholesterol, plasma membrane protrusions and human pathology. Traffic. 2001;2(2):82-91.
Glumac PM, LeBeau AM. The role of CD133 in cancer: a concise review. Clin. Transl. Med. 2018;7(1):18. doi: https://doi.org/10.1186/s40169-018-0198-1.
Jang JW, Song Y, Kim SH, Kim J, Seo HR. Potential mechanisms of CD133 in cancer stem cells. Life Sci. 2017;184:25-29.
Kim YS, Kaidina AM, Chiang JH, Yarygin KN, Lupatov AY. Cancer stem cell molecular markers verified in vivo. Biomed. Khim. 2016;62(3):228-238.
Mak AB, Nixon AM, Kittanakom S, Stewart JM, Chen GI, Curak J, Gingras AC, Mazitschek R, Neel BG, Stagljar I, Moffat J. Regulation of CD133 by HDAC6 promotes β-catenin signaling to suppress cancer cell differentiation. Cell Rep. 2012;2(4):951-963.
Röper K, Corbeil D, Huttner WB. Retention of prominin in microvilli reveals distinct cholesterol-based lipid micro-domains in the apical plasma membrane. Nat. Cell Biol. 2000;2(9):582-592.
Zacchigna S, Oh H, Wilsch-Bräuninger M, Missol-Kolka E, Jászai J, Jansen S, Tanimoto N, Tonagel F, Seeliger M, Huttner WB, Corbeil D, Dewerchin M, Vinckier S, Moons L, Carmeliet P. Loss of the cholesterol-binding protein prominin-1/CD133 causes disk dysmorphogenesis and photoreceptor degeneration. J. Neurosci. 2009;29(7):2297-2308.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Kletochnye Tekhnologii v Biologii i Meditsine, No. 2, pp. 84-89, June, 2019
Rights and permissions
About this article
Cite this article
Gisina, A.M., Kim, Y.S., Potashnikova, D.M. et al. Proliferative Activity of Colorectal Cancer Cells with Different Levels of CD133 Expression. Bull Exp Biol Med 167, 541–545 (2019). https://doi.org/10.1007/s10517-019-04569-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-019-04569-y