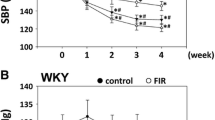
Changes in BP and HR were assessed after exposures increasing activity of angiotensin-converting enzyme: ionizing radiation, NO synthase inhibitor (L-NAME), and dexamethasone. Effects of dihydroquercetin and angiotensin-converting enzyme inhibitor enalapril on activity of this enzyme, BP, and HR were also evaluated under these exposures. Wistar male rats were subjected to X-ray irradiation in a dose of 2.5 Gy. Angiotensin-converting enzyme activity in the aorta sections was determined by Hip-His-Leu hydrolysis. BP and HR were recorded using a non-invasive tail-cuff method and PowerLab 8/35 software. BP and HR were not altered with the increase in activity of angiotensin-converting enzyme after irradiation. In case of prolonged (7 days) treatment with NO synthase inhibitor and dexamethasone, the increase in enzyme activity was accompanied by elevation of BP and, in the case of NO synthase inhibitor, HR reduction. Dihydroquercetin normalized the enzyme activity and lowered BP, but not to the normal level. Enalapril normalized BP, increased by NO synthase inhibitor solution intake; at the same time, the angiotensinconverting enzyme activity decreased more than 2-fold in comparison with the normal.
Similar content being viewed by others
References
Korystova AF, Kublik LN, Kim YA, Levitman MK, Shaposhnikova VV, Korystov YN. Dihydroquercetin and Fucoidin Inhibit the Increase of Angiotensin-Converting Enzyme Activity in the Rat Aorta after Irradiation. Bull. Exp. Biol. Med. 2018;165(3):360-363.
Korystova AF, Kublik LN, Levitman MK, Samokhvalova TV, Shaposhnikova VV, Korystov YN. Ionizing Radiation Enhances Activity of Angiotensin-Converting Enzyme in Rat Aorta. Bull. Exp. Biol. Med. 2018;165(2):216-219.
Orlov VA, Gilyarevsky SR, Urusbieva DM, Daurbekova LV. Adverse effects of ACE inhibitors and cardiovascular treatment tactics. Ross. Kardiol. Zh. 2005;10(3):79-90. Russian.
Ackermann A, Fernández-Alfonso MS, Sánchez de Rojas R, Ortega T, Paul M, González C. Modulation of angiotensin-converting enzyme by nitric oxide. Br. J. Pharmacol. 1998;124(2):291-298.
Arutyunyan TV, Korystova AF, Kublik LN, Levitman MKh, Shaposhnikova VV, Korystov YN. Effects of taxifolin on the activity of angiotensin-converting enzyme and reactive oxygen and nitrogen species in the aorta of aging rats and rats treated with the nitric oxide synthase inhibitor and dexamethasone. Age (Dordr). 2013;35(6):2089-2097.
Bader M. Tissue renin-angiotensin-aldosterone systems: Targets for pharmacological therapy. Annu. Rev. Pharmacol. Toxicol. 2010;50:439-465.
Fitzgerald S.M, Brands M.W. Hypertension in L-NAMEtreated diabetic rats depends on an intact sympathetic nervous system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282(4):R1070-R1076.
Kim S, Iiwao H. Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacol. Rev. 2000;52(1):12-34.
Linz W, Wohlfart P, Schölkens BA, Malinski T, Wiemer G. Interactions among ACE, kinins and NO. Cardiovasc. Res. 1999;43(3):549-561.
Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am. J. Physiol. Cell Physiol. 2007;292(1):C82-C97.
Touyz RM. Blood Pressure Regulation and Pathology. Cellular and Molecular Pathobiology of Cardiovascular Disease. Willis MS, Homeister JW, Stone JR, eds. Glasgow, 2014. P. 257-275.
Schäfer SC, Wallerath T, Closs EI, Schmidt C, Schwarz PM, Förstermann U, Lehr HA. Dexamethasone suppresses eNOS and CAT-1 and induces oxidative stress in mouse resistance arterioles. Am. J. Physiol. Heart Circ. Physiol. 2005;288(1):H436-H444.
Zanzinger J, Czachurski J, Seller H. Inhibition of sympathetic vasoconstriction is a major principle of vasodilation by nitric oxide in vivo. Circ. Res. 1994;75(6):1073-1077.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Byulleten’ Eksperimental’noi Biologii i Meditsiny, Vol. 166, No. 7, pp. 36-40, July, 2018
Rights and permissions
About this article
Cite this article
Korystova, A.F., Kublik, L.N., Levitman, M.K. et al. Blood Pressure Changes After Exposures Increasing Angiotensin-Converting Enzyme Activity and After Its Normalization with Dihydroquercetin in Male Wistar Rats. Bull Exp Biol Med 166, 31–34 (2018). https://doi.org/10.1007/s10517-018-4282-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-018-4282-8