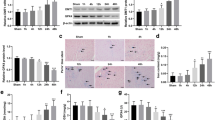
We studied the expression of Hif-1α, Nf-κb, and Vegf genes in the liver and serum levels of HIF-1α, erythropoietin, VEGF, TGF-β, 8-isoprostane, and corticosterone in Wistar rats with different resistance to hypoxia in 5 and 90 min after acute exposure to hypobaric hypoxia. In 5 min after hypoxic exposure, Hif-1α expression in the liver and serum levels of erythropoietin, VEGF, and TGF-β in high-resistant rats were higher than in low-resistant animals. In highresistant rats, the increment in expression of Nf-κb gene responsible for the control over the inflammatory processes was more pronounced than in low-resistant animals. In 90 min after hypoxic exposure, the serum levels of HIF-1α, erythropoietin, VEGF, and TGF-β returned to normal in high-resistant rats, while in low-resistant animals, an increase in 8-isoprostane and TGF-β concentrations was observed. The rats with different resistance to hypoxia were characterized by different changes in biomolecular parameters determining predilection to inflammatory diseases.
Similar content being viewed by others
References
Agadzhanyan NA, Khachatur’yan ML, Panchenko LA. Effect of acute hypoxia on resistance to hypoxia in rats. Bull. Exp. Biol. Med. 1999;127(6):567-570.
Luk’yanova LD. Molecular mechanisms of tissue hypoxia and adaptation. Fiziol. Zh. 2003;49(3):17-35. Russian.
Lukyanova LD, Kirova YI. Effect of hypoxic preconditioning on free radical processes in tissues of rats with different resistance to hypoxia. Bull. Exp. Biol. Med. 2011;151(3):292-296.
Belaiba RS, Bonello S, Zähringer C, Schmidt S, Hess J, Kietzmann T, Görlach A. Hypoxia up-regulates hypoxia-inducible factor-1alpha transcription by involving phosphatidylinositol 3-kinase and nuclear factor kappaB in pulmonary artery smooth muscle cells. Mol. Biol. Cell. 2007;18(12):4691-4697.
Fandrey J. Oxygen-dependent and tissue-specific regulation of erythropoietin gene expression. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286(6):R977-R988.
Ghosh D, Kumar R, Pal K. Individual variation in response to simulated hypoxic stress of rats. Indian J. Exp. Biol. 2012; 50(10):744-748.
Hirota K. Involvement of hypoxia-inducible factors in the dysregulation of oxygen homeostasis in sepsis. Cardiovasc. Hematol. Disord. Drug Targets. 2015;15(1):29-40.
Jain K, Suryakumar G, Prasad R, Ganju L. Upregulation of cytoprotective defense mechanisms and hypoxia-responsive proteins imparts tolerance to acute hypobaric hypoxia. High Alt. Med. Biol. 2013;14(1):65-77.
Jiang Y, Dai A, Li Q, Hu R. Hypoxia induces transforming growth factor-beta1 gene expression in the pulmonary artery of rats via hypoxia-inducible factor-1alpha. Acta Biochim. Biophys. Sin. (Shanghai). 2007;39(1):73-80.
Liu RM, Desai LP. Recirpocal regulation of TGF-β and reactive oxygen species: a perverse cycle for fibrosis. Redox Biol. 2015;6:565-577.
Padhy G, Sethy NK, Ganju L, Bhargava K. Abundance of plasma antioxidant proteins confers tolerance to acute hypobaric hypoxia exposure. High Alt. Med. Biol. 2013;14(3):289-297.
Pialoux V, Hanly PJ, Foster GE, Brugniaux JV, Beaudin AE, Hartmann SE, Pun M, Duggan CT, Poulin MJ. Effects of exposure to intermittent hypoxia on oxidative stress and acute hypoxic ventilatory response in humans. Am. J. Respir. Crit. Care Med. 2009;180(10):1002-1009.
Sánchez-Elsner T, Ramírez JR, Sanz-Rodriguez F, Varela E, Bernabéu C, Botella LM. A cross-talk between hypoxia and TGF-beta orchestrates erythropoietin gene regulation through SP1 and Smads. J. Mol. Biol. 2004;336(1):9-24.
Semenza G.L. Life with oxygen. Science. 2007;318:62-64.
Stroka DM, Burkhardt T, Desbaillets I, Wenger RH, Neil DA, Bauer C, Gassmann M, Candinas D. HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J. 2001;15(13):2445-2453.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Byulleten’ Eksperimental’noi Biologii i Meditsiny, Vol. 165, No. 6, pp. 742-747, June, 2018
Rights and permissions
About this article
Cite this article
Dzhalilova, D.S., Diatroptov, M.E., Tsvetkov, I.S. et al. Expression of Hif-1α, Nf-κb, and Vegf Genes in the Liver and Blood Serum Levels of HIF-1α, Erythropoietin, VEGF, TGF-β, 8-Isoprostane, and Corticosterone in Wistar Rats with High and Low Resistance to Hypoxia. Bull Exp Biol Med 165, 781–785 (2018). https://doi.org/10.1007/s10517-018-4264-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-018-4264-x