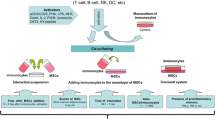
Analysis of changes in lymphocyte subpopulations during co-culturing with multipotent mesenchymal stromal cells (MSC) revealed two distinct MSC groups: one group (A) increased HLA-DR expression on lymphocytes during co-culturing and the other (B) did not change it in comparison with lymphocyte monoculture. In stromal cells interacting with lymphocytes, expression of HLA-DR molecules was initiated, but only in samples that induced enhanced expression on lymphocytes and irrespectively of whether allogeneic or autologous lymphocytes were used for co-culturing with MSC. In group A, the relative expression of IDO1 significantly increased in comparison with group B. The revealed individual differences in MSC can explain why not all MSC samples are effective in the treatment of autoimmune diseases, acute “graft—versus—host” disease, and other pathologies.
Similar content being viewed by others
References
Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005; 105(4):1815-1822.
Castro-Manrreza ME, Montesinos JJ. Immunoregulation by mesenchymal stem cells: biological aspects and clinical applications. J. Immunol. Res. 2015;2015. ID 394917. doi: https://doi.org/10.1155/2015/394917.
Jones BJ, McTaggart SJ. Immunosuppression by mesenchymal stromal cells: from culture to clinic. Exp. Hematol. 2008; 36(6):733-741.
Kapranov NM, Davydova YO, Galtseva IV, Petinati NA, Drize NI, Kuzmina LA, Parovichnikova EN, Savchenko VG. Effect of priming of multipotent mesenchymal stromal cells with interferon g on their immunomodulating properties. Biochemistry (Mosc). 2017;82(10):1158-1168.
Kapranov NM, Davydova JuO, Petinati NA, Bakshinskayte MV, Galtseva IV, Drize NI, Kuzmina LA, Parovichnikova EN, Savchenko VG. Alterations in multipotent mesenchymal stromal cells properties: in vitro model of their interactions with allogeneic lymphocytes. Cell. Ther. Transplant. 2016;5(3):39-41.
Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, Santarlasci V, Mazzinghi B, Pizzolo G, Vinante F, Romagnani P, Maggi E, Romagnani S, Annunziato F. Role for interferon-g in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24(2):386-398.
Le Blanc K, Rasmusson I, Götherström C, Seidel C, Sundberg B, Sundin M, Rosendahl K, Tammik C, Ringdén O. Mesenchymal stem cells inhibit the expression of CD25 (interleukin-2 receptor) and CD38 on phytohaemagglutinin-activated lymphocytes. Scand. J. Immunol. 2004;60(3):307-315.
Mahnke YD, Brodie TM, Sallusto F, Roederer M, Lugli E. The who’s who of T-cell differentiation: Human memory T-cell subsets. Eur. J. Immunol. 2013;43(11):2797-2809.
Petinati NA, Kapranov NM, Bigil’deev AE, Popova MD, Davydova YO, Gal’tseva IV, Drize NI, Kuz’mina LA, Parovichnikova EN, Savchenko VG. Changing the properties of multipotent mesenchymal stromal cells by IFNg administration. Bull. Exp. Biol. Med. 2017;163(2):230-234.
Petinati N, Shipounova I, Sats N, Bigildeev A, Drize N, Kuzmina L, Parovichnikova E, Savchenko V. Alterations between effective and ineffective multipotent mesenchymal stromal cells used for acute graft versus host disease prophylaxis. Med. Res. Arch. 2016;4(1):1-22.
Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C (T) method. Nat. Protoc. 2008;3(6):1101-1108.
Wang M, Windgassen D, Papoutsakis ET. Comparative analysis of transcriptional profiling of CD3+, CD4+ and CD8+ T cells identifies novel immune response players in T-cell activation. BMC Genomics. 2008;9:225. doi: https://doi.org/10.1186/1471-2164-9-225.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Kletochnye Tekhnologii v Biologii i Meditsine, No. 2, pp. 129-134, June, 2018
Rights and permissions
About this article
Cite this article
Kapranov, N.M., Davydova, Y.O., Gal’tseva, I.V. et al. Individual Differences of Multipotent Mesenchymal Stromal Cells Manifesting in during Interaction with Lymphocytes. Bull Exp Biol Med 165, 584–588 (2018). https://doi.org/10.1007/s10517-018-4218-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-018-4218-3