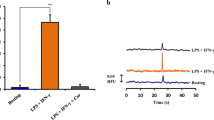
The effects of glufimet and phenibut (glutamic acid and GABA derivatives, respectively) on concentration of inducible NO synthase and cGMP in LPS-activated mouse peritoneal macrophages and on NO end products in their culture medium were examined in vitro and ex vivo. Addition of LPS into culture medium elevated concentration of NO metabolites in this medium and increased concentration of inducible NO synthase and cGMP in the lysates of peritoneal macrophages, whereas incubation of the cells with examined agents applied at concentration of 10—5 M diminished these indices. Similar results were obtained with intraperitoneal injection of LPS, glufimet, and phenibut. In culture medium containing peritoneal macrophages from the mice injected with LPS (100 μg/kg), the concentrations of inducible NO synthase and cGMP as well as the total concentration of nitrite and nitrate ions increased, whereas in culture medium with the cells from LPS-exposed mice treated with glufimet (28.7 mg/kg) and phenibut (50 mg/kg) these indices significantly decreased.
Similar content being viewed by others
References
Tyurenkov IN, Samotrueva MA, Luzhnova SA. Modifying influence of GABAB receptors agonists on interleukin levels in case of experimental immunopathology. Tsitokiny Vospalenie 2014;13(4):42-45. Russian.
Busnardo C, Crestani CC, Tavares RF, Resstel LB, Correa FM. Cardiovascular responses to L-glutamate microinjection into the hypothalamic paraventricular nucleus are mediated by a local nitric oxide-guanylate cyclase mechanism. Brain Res 2010;1344:87-95.
Chen HJ, Spiers JG, Sernia C, Lavidis NA. Response of the nitrergic system to activation of the neuroendocrine stress axis. Front. Neurosci. 2015;9:3. doi: https://doi.org/10.3389/fnins.2015.00003.
Floden AM, Li S, Combs CK. Beta-amyloid-stimulated microglia induce neuron death via synergistic stimulation of tumor necrosis factor alpha and NMDA receptors. J. Neurosci. 2005;25(10):2566-2575.
Foreman MA, Gu Y, Howl JD, Jones S, Publicover SJ. Group III metabotropic glutamate receptor activation inhibits Ca2+ influx and nitric oxide synthase activity in bone marrow stromal cells. J. Cell. Physiol. 2005;204(2):704-713.
Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur. Heart J. 2012;33(7):829-837.
Fortier AH, Hoover DL, Nacy CA. Intracellular replication of eishmania tropica in mouse peritoneal macrophages: amastigote infection of resident cells and inflammatory exudate macrophages. Infect. Immun. 1982;38(3):1304-1308.
French SJ, Ritson GP, Hidaka S, Totterdell S. Nucleus accumbens nitric oxide immunoreactive interneurons receive nitric oxide and ventral subicular afferents in rats. Neuroscience. 2005;135(1):121-131.
Harvey BH, Oosthuizen F, Brand L, Wegener G, Stein DJ. Stress-restress evokes sustained iNOS activity and altered GABA levels and NMDA receptors in rat hippocampus. Psychopharmacology. (Berl). 2004;175(4):494-502.
Kamran M, Bahrami A, Soltani N, Keshavarz M, Farsi L. GABA-induced vasorelaxation mediated by nitric oxide and GABAA receptor in non diabetic and streptozotocin-induced diabetic rat vessels. Gen. Physiol. Biophys. 2013;32(1):101-106.
Lee M, Schwab C, McGeer PL. Astrocytes are GABAergic cells that modulate microglial activity. Glia 2011;59(1):152-165.
Loane DJ, Stoica BA, Byrnes KR, Jeong W, Faden AI. Activation of mGluR5 and inhibition of NADPH oxidase improves functional recovery after traumatic brain injury. J. Neurotrauma. 2013;30(5):403-412.
Mauriz JL, Matilla B, Culebras JM, González P, González-Gallego J. Dietary glycine inhibits activation of nuclear factor kappa B and prevents liver injury in hemorrhagic shock in the rat. Free Radic. Biol. Med. 2001;31(10):1236-1244.
Vodovotz Y, Kwon NS, Pospischil M, Manning J, Paik J, Nathan C. Inactivation of nitric oxide synthase after prolonged incubation of mouse macrophages with IFN-gamma and bacterial lipopolysaccharide. J. Immunol. 1994;152(8):4110-4118.
Yao HH, Ding JH, Zhou F, Wang F, Hu LF, Sun T, Hu G. Enhancement of glutamate uptake mediates the neuroprotection exerted by activating group II or III metabotropic glutamate receptors on astrocytes. J. Neurochem. 2005;92(4):948-961.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Byulleten’ Eksperimental’noi Biologii i Meditsiny, Vol. 164, No. 8, pp. 205-208, August, 2017
Rights and permissions
About this article
Cite this article
Borisov, A.V., Prokofiev, I.I., Mokrousov, I.S. et al. Inhibition of the Expression of Inducible NO Synthase by Neuroactive Amino Acid Derivatives Phenibut and Glufimet In Vitro and Ex Vivo . Bull Exp Biol Med 164, 177–180 (2017). https://doi.org/10.1007/s10517-017-3952-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-017-3952-2