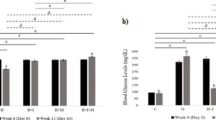
We studied the effects of a melatonin—aluminum oxide—polymethylsiloxane complex (complex M) on the expression of apoptosis regulators Bcl-2 and Bad in the liver of homozygous db/db BKS.Cg-Dock7m+/+Leprdb/J mice with obesity and type 2 diabetes. Complex M or placebo was administered daily through the gastric tube during weeks 8-16 of life. In mice with type 2 diabetes mellitus receiving placebo, enhanced immunohistochemical reactions for proapoptotic Bad protein and weak response for anti-apoptotic Bcl-2 protein were observed. Administration of complex M shifted the ratio of apoptosis regulators: the area of Bcl-2 expression significantly increased and against the background of reduced Bad expression area. These findings attest to antiapoptotic effect of complex M in the liver on the model of type 2 diabetes mellitus.
Similar content being viewed by others
References
Konenkov VI, Klimontov VV, Michurina SV, Prudnikova MA, Ishenko IJu. Melatonin and diabetes: from pathophysiology to the treatment perspectives. Sakhar. Diabet. 2013;(2):11-16. Russian.
Michurina SV, Ishchenko IY, Arkhipov SA, Klimontov VV, Rachkovskaya LN, Konenkov VI, Zavyalov EL. Effects of Melatonin, Aluminum Oxide, and Polymethylsiloxane Complex on the Expression of LYVE-1 in the Liver of Mice with Obesity and Type 2 Diabetes Mellitus. Bull. Exp. Biol. Med. 2016;162(2):269-272.
Michurina SV, Rachkovskaja LN, Ishchenko IJ, Rachkovskij EE, Klimontov VV, Konenkov VI. Patent RU No. 2577580. Alumina-based porous sorbent with chronotropic properties. Bull. No. 8. Published March 20, 2016.
Agil A, El-Hammadi M, Jiménez-Aranda A, Tassi M, Abdo W, Fernández-Vázquez G, Reiter RJ. Melatonin reduces hepatic mitochondrial dysfunction in diabetic obese rats. J. Pineal Res. 2015;59(1):70-79.
de Luxán-Delgado B, Potes Y, Rubio-González A, Caballero B, Solano JJ, Fernández-Fernández M, Bermúdez M, Rodrigues Moreira Guimarães M, Vega-Naredo I, Boga JA, Coto-Montes A. Melatonin reduces endoplasmic reticulum stress and autophagy in liver of leptin-deficient mice. J. Pineal. Res. 2016;61(1):108-123.
Hatzis G, Ziakas P, Kavantzas N, Triantafyllou A, Sigalas P, Andreadou I, Ioannidis K, Chatzis S, Filis K, Papalampros A, Sigala F. Melatonin attenuates high fat diet-induced fatty liver disease in rats. World J. Hepatol. 2013;5(4):160-169.
Hu S, Yin S, Jiang X, Huang D, Shen G. Melatonin protects against alcoholic liver injury by attenuating oxidative stress, inflammatory response, and apoptosis. Eur. J. Pharmacol. 2009;616(1-3):287-292.
Kireev R, Bitoun S, Cuesta S, Tejerina A, Ibarrola C, Moreno E, Vara E, Tresguerres JA. Melatonin treatment protects liver of Zucker rats after ischemia/reperfusion by diminishing oxidative stress and apoptosis. Eur. J. Pharmacol. 2013;701(1-3):185-193.
Michurina SV, Ishenko IJ, Klimontov VV, Archipov SA, Myakina NE, Cherepanova MA, Zavjalov EL, Koncevaya GV, Konenkov VI. Linagliptin alleviates fatty liver disease in diabetic db/db mice. World J. Diabetes. 2016;7(19):534-546.
Schuppan D, Schattenberg JM. Non-alcoholic steatohepatitis: pathogenesis and novel therapeutic approaches. J. Gastroenterol. Hepatol. 2013;28(Suppl. 1):68-76.
Sheen JM, Chen YC, Hsu MH, Tain YL, Huang YH, Tiao MM, Li SW, Huang LT. Melatonin alleviates liver apoptosis in bile duct ligation young rats. Int. J. Mol. Sci. 2016;17(8):pii: E1365. doi: 10.3390/ijms17081365.
Stacchiotti A, Favero G, Lavazza A, Golic I, Aleksic M, Korac A, Rodella LF, Rezzani R. Hepatic macrosteatosis is partially converted to microsteatosis by melatonin supplementation in ob/ob mice non-alcoholic fatty liver disease. PLoS One. 2016;11(1):e0148115. doi: https://doi.org/10.1371/journal.pone.0148115.
Sun H, Wang X, Chen J, Song K, Gusdon AM, Li L, Bu L, Qu S. Melatonin improves non-alcoholic fatty liver disease via MAPK-JNK/P38 signaling in high-fat-diet-induced obese mice. Lipids Health Dis. 2016;15(1):202.
Zavodnik IB, Lapshina EA, Cheshchevik VT, Dremza IK, Kujawa J, Zabrodskaya SV, Reiter RJ. Melatonin and succinate reduce rat liver mitochondrial dysfunction in diabetes. J. Physiol. Pharmacol. 2011;62(4):421-427.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Byulleten’ Eksperimental’noi Biologii i Meditsiny, Vol. 164, No. 8, pp. 192-196, August, 2017
Rights and permissions
About this article
Cite this article
Michurina, S.V., Ischenko, I.Y., Arkhipov, S.A. et al. Melatonin—Aluminum Oxide—Polymethylsiloxane Complex on Apoptosis of Liver Cells in a Model of Obesity and Type 2 Diabetes Mellitus. Bull Exp Biol Med 164, 165–169 (2017). https://doi.org/10.1007/s10517-017-3949-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-017-3949-x