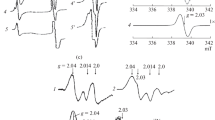
The study showed that dinitrosyl iron complex (NO)2Fe(RS)2 containing the thiolate ligands, which is the basic physiological donor of NO, can transfer NO to other molecule only at the moment of rearrangement. This rearrangement can occur during interaction of the complex with more effective iron chelators than the thiolate ligands. In the absence of NO trap, a new complex is formed with a new ligand. NO transfer to a trap can also occur under the action of the agents such as mercury salts or ROS, which interact with the thiolate ligands. Probably, the ligands in the dinitrosyl iron complexes are the structures responsible for interaction of these complexes with physiological targets and for specificity and effectiveness of this interaction.
Similar content being viewed by others
References
Titov VY, Kosenko OV, Starkova ES, Kondratov GV, Borkhunova EN, Petrov VA, Osipov AN. Enzymatic Sensor Detects Some Forms of Nitric Oxide Donors Undetectable by Other Methods in Living Tissues. Bull. Exp. Biol. Med. 2016;162(1):107-110.
Titov VY, Osipov AN, Kreinina MV, Vanin AF. Features of the metabolism of nitric oxide in normal state and inflammation. Biophysics. 2013;58(5):676-688.
Balazy M, Kaminski PM, Mao K, Tan J, Wolin MS. S-nitroglutathione, a product of the reaction between peroxynitrite and glutathione that generates nitric oxide. J. Biol. Chem. 1998;273(48):32 009-32 015.
Borodulin RR, Kubrina LN, Mikoyan VD, Poltorakov AP, Shvydkiy VО, Burbaev DSh, Serezhenkov VA, Yakhontova ER, Vanin AF. Dinitrosyl iron complexes with glutathione as NO and NO+ donors. Nitric Oxide. 2013;29:4-16.
Bruning-Fann CS, Kaneene JB. The effects of nitrate, nitrite, and N-nitroso compounds on animal health. Vet. Hum. Toxicol. 1993;35(3):237-253.
Gladwin MT, Ognibene FP, Shelhamer JH, Pease-Fye ME, Noguchi CT, Rodgers GP, Schechter AN. Nitric oxide transport on sickle cell hemoglobin: where does it bind. Free Radic. Res. 2001;35(2):175-180.
Gladwin MT, Shelhamer JH, Schechter AN, Pease-Fye ME, Waclawiw MA, Panza JA, Ognibene FP, Cannon RO 3rd . Role of circulating nitrite and S-nitrosohemoglobin in the regulation of regional blood flow in humans. Proc. Natl Acad. Sci. USA. 2000;97(21):11,482-11,487.
Preik-Steinhoff H, Kelm M. Determination of nitrite in human blood by combination of a specific sample preparation with high-performance anion-exchange chromatography and electrochemical detection. J. Chromatogr. B Biomed. Appl. 1996;685(2):348-352.
Rassaf T, Preik M, Kleinbongard P, Lauer T, Heiss C, Strauer BE, Feelisch M, Kelm M. Evidence for in vivo transport of bioactive nitric oxide in human plasma. J. Clin. Invest. 2002;109(9):1241-1248.
Severina IS, Bussygina OG, Pyatakova NV, Malenkova IV, Vanin AF. Activation of soluble guanylate cyclase by NO donors-S-nitrosothiols, and dinitrosyl iron complexes with thiol-containing ligands. Nitric Oxide. 2003;8(3):155-163.
Shumaev KB, Gubkin AA, Serezhenkov VA, Lobysheva II, Kosmachevskaya OV, Ruuge EK, Lankin VZ, Topunov AF, Vanin AF. Interaction of reactive oxygen and nitrogen species with albumin- and methemoglobin-bound dinitrosyl iron complexes. Nitric Oxide. 2008;18(1):37-46.
Titov VY. The enzymatic technologies open new possibilities for studying nitricoxide (NO) metabolism in living systems. Curr. Enzym. Inhib. 2011;7(1):56-70.
Titov VY, Osipov AN. Nitrite and nitroso compounds can serve as specific catalase inhibitors. Redox Rep. 2017;22(2):91-97.
Vanin AF. Dinitrosyl iron complexes with thiolate ligands: physico-chemistry, biochemistry and physiology. Nitric Oxide. 2009;21(1):1-13.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Byulleten’ Eksperimental’noi Biologii i Meditsiny, Vol. 163, No. 6, pp. 691-695, June, 2017
Rights and permissions
About this article
Cite this article
Titov, V.Y., Dolgorukova, A.M., Petrov, V.A. et al. Selectivity in Physiological Action of Nitric Oxide: A Hypothetical Mechanism. Bull Exp Biol Med 163, 726–730 (2017). https://doi.org/10.1007/s10517-017-3890-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-017-3890-z