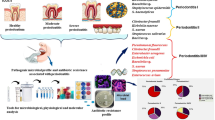
Biofilm of the gingival sulcus from 22 patients with type 2 diabetes mellitus and periodontitis, 30 patients with periodontitis not complicated by diabetes mellitus (reference group), and 22 healthy volunteers without signs of gingival disease (control group) was studied by quantitative PCR. Quantitative analysis for the content of P. gingivalis, T. forsythia, A. ctinomycetemcomitans, T. denticola, P. intermedia, F. nucleatum/periodonticum, and P. endodontalis in the dental plaque was performed with a Dentoscreen kit. The presence of other bacterial groups was verified by metagenomic sequencing of the 16S rRNA gene to evaluate some specific features of the etiological factor for periodontitis in type 2 diabetes mellitus. Specimens of the Porphiromonadaceae and Fusobacteriaceae families were characterized by an extremely high incidence in combined pathology. The amount of Sphingobacteriaceae bacteria in the biofilm was shown to decrease significantly during periodontitis. Metagenomic analysis confirmed the pathogenic role of microbiota in combined pathology, as well as the hypothesis on a possible influence of periodontitis on the course and development of type 2 diabetes mellitus.
Similar content being viewed by others
References
Bogomolov MV. Periodontitis as a nonspecific complication of diabetes mellitus. Methods of prevention. Russ. Med. Zh. 2011;19(13):829-831. Russian.
Ippolitov EV, Didenko LV, Tzarev VN. The characteristics of morphology of biofilm of periodontium under inflammatory diseases of gums (chronic catarrhal gingivitis, chronic periodontitis, Candida-associated periodontitis) according results of electronic microscopy. Klin. Lab. Diagnost. 2015;60(12):59-64. Russian.
Nikolaeva EN, Tsarev VN, Ippolitov EV. Parodontopathogenic bacteria as indicators of the risk of periodontitis (part II). Stomatologiya dlya Vsekh. 2011;(4):4-7.
Rebrikov DV. Real-Time PCR. Moscow, 2014. Russian.
Lalla E, Papapanou PN. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat. Rev. Endocrinol. 2011;7(12):738-748.
Matsushita K, Hamaguchi M, Hashimoto M, Yamazaki M, Yamazaki T, Asai K, Yamori M, Bessho K, Toda H, Hasegawa G, Nakamura N, Fukui M. The novel association between red complex of oral microbe and body mass index in healthy Japanese: a population based cross-sectional study. J. Clin. Biochem. Nutr. 2015;57(2):135-139.
Patil VS, Patil VP, Gokhale N, Acharya A, Kangokar P. Chronic periodontitis in type 2 diabetes mellitus: oxidative stress as a common factor in periodontal tissue injury. J. Clin. Diagn. Res. 2016;10(4):BC12-BC16.
Rôças IN, Siqueira JF Jr, Santos KR, Coelho AM. “Red complex” (Bacteroides forsythus, Porphyromonas gingivalis, and Treponema denticola) in endodontic infections: a molecular approach. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001;91(4):468-471.
Straczkowski M, Kowalska I. The role of skeletal muscle sphingolipids in the development of insulin resistance. Rev. Diabet. Stud. 2008;5(1):13-24.
Tilg H, Moschen AR. Insulin resistance, inflammation, and non-alcoholic fatty liver disease. Trends Endocrinol. Metab. 2008;19(10):371-379.
Wang GP. Defining functional signatures of dysbiosis in periodontitis progression. Genome Med. 2015;7(1):40.
Zhou M, Rong R, Munro D, Zhu C, Gao X, Zhang Q, Dong Q. Investigation of the effect of type 2 diabetes mellitus on subgingival plaque microbiota by high-throughput 16S rDNA pyrosequencing. PLoS One. 2013;8(4):e61516. doi: https://doi.org/10.1371/journal.pone.0061516.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Byulleten’ Eksperimental’noi Biologii i Meditsiny, Vol. 163, No. 6, pp. 682-686, June, 2017
Rights and permissions
About this article
Cite this article
Babaev, E.A., Balmasova, I.P., Mkrtumyan, A.M. et al. Metagenomic Analysis of Gingival Sulcus Microbiota and Pathogenesis of Periodontitis Associated with Type 2 Diabetes Mellitus. Bull Exp Biol Med 163, 718–721 (2017). https://doi.org/10.1007/s10517-017-3888-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-017-3888-6