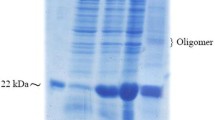
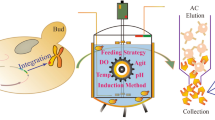
Genetic constructs with different leader sequences for intra- and extracellular expression of the target protein were generated and an original method for effective selection of clones with maximum expression was developed. For intracellular expression in the Pichia pastoris system, seprin content in cells was 6 mg/liter.
Similar content being viewed by others
References
Isaev VA, Rudenskaja GN, Kostin NN, Sergeeva EN, Statsenko IV, Kolesnikova TJ. Patent RF No. 2560264. Method of production of recombinant serine protease inhibitor of King Crab. Bull. No. 23. Published August 20, 2015.
Apte-Deshpande A, Rewanwar S, Kotwal P, Raiker VA, Padmanabhan S. Efficient expression and secretion of recombinant human growth hormone in the methylotrophic yeast Pichia pastoris: potential applications for other proteins. Biotechnol. Appl. Biochem. 2009;54(4):197-205.
Balashova MV, Lyutova LV, Rudenskaya YA, Isaev VA, Andina SS, Koslov LV, Rudenskaya GN. Anticoagulant and anticompliment effect of an endogenous inhibitor from hepatopancreas of red king crab (Paralithosed camtshaticus). Biol. Chem. 2011;5(3):268-275.
Gettins PG. Serpin structure, mechanism, and function. Chem. Rev. 2002;102(12):4751-4804.
Kuznetsov SA, Kuranova IP, Golyshev SA, Rudenskaya YuA, Isaev VA, Rudenskaya GN. A novel endogenous inhibitor from the hepatopancreas of the kamchatka crab Paralithodes camtschaticus. Russ. J. Bioorgan. Chem. 2012;38(3):290-297.
Pemberton PA, Bird PI. Production of serpins using yeast expression systems. Methods. 2004;32(2):185-190.
Shukla AK, Reinhart C, Michel H. Dimethylsulphoxide as a tool to increase functional expression of heterologously produced GPCRs in mammalian cells. FEBS Lett. 2006;580(17):4261-4265.
Silverman GA, Bird PI, Carrell RW, Church FC, Coughlin PB, Gettins PG, Irving JA, Lomas DA, Luke CJ, Moyer RW, Pemberton PA, Remold-O’Donnell E, Salvesen GS, Travis J, Whisstock JC. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J. Biol. Chem. 2001;276(36):33,293-33,296.
Wu S, Letchworth GJ. High efficiency transformation by electroporation of Pichia pastoris pretreated with lithium acetate and dithiothreitol. Biotechniques. 2004;36(1):152-154.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Byulleten’ Eksperimental’noi Biologii i Meditsiny, Vol. 163, No. 2, pp. 171-175, February, 2017
Rights and permissions
About this article
Cite this article
Kostin, N.N., Ponomarenko, N.A., Isaev, V.A. et al. Preparation of Recombinant Serpin from Red King Crab Paralithodes сamtschaticus for Biomedical Research Purposes. Bull Exp Biol Med 163, 210–213 (2017). https://doi.org/10.1007/s10517-017-3768-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-017-3768-0