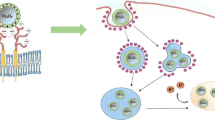
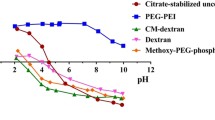
The behavior of magnetite nanoparticles was studied in the cell chip microcapillaries. No aggregation of magnetite nanoparticles under conditions of long-term circulation was noted. Biodistribution and toxicity of magnetite nanoparticles (14 nm) and aminated magnetite after their intragastric administration to mice were studied in vivo. According to mass spectrometry and microscopy data, accumulation of nanoparticles occurred mainly in the liver cells.
Similar content being viewed by others
References
B. Ataç, I. Wagner, R. Horland, R. Lauster, U. Marx, A. G. Tonevitsky, R. P. Azar, and G. Lindner, Skin and hair on-achip: in vitro skin models versus ex vivo tissue maintenance with dynamic perfusion, Lab. Chip, 13, No. 18, 3555–3561 (2013).
S. N. Bhatia and D. E. Ingber, Microfluidic organs-on-chips, Nat. Biotechnol., 32, No. 8, 760–772 (2014).
A. K. Bordbar, A. A. Rastegari, R. Amiri, E. Ranjbakhsh, M. Abbasi, and A. R. Khosropour, Characterization of modified magnetite nanoparticles for albumin immobilization, Biotechnol. Res. Int., doi: 10.1155/2014/705068 (2014).
B. A. Katsnelson, L. I. Privalova, M. P. Sutunkova, V. B. Gurvich, N. V. Loginova, I. A. Minigalieva, E. P. Kireyeva, V. Y. Shur, E. V. Shishkina, Y. B. Beikin, O. H. Makeyev, and I. E. Valamina, Some inferences from in vivo experiments with metal and metal oxide nanoparticles: the pulmonary phagocytosis response, subchronic systemic toxicity and genotoxicity, regulatory proposals, searching for bioprotectors (a self-overview), Int. J. Nanomedicine, 10, 3013–3029 (2015).
G. Langley, C. P. Austin, A. K. Balapure, L. S. Birnbaum, J. R. Bucher, J. Fentem, S. C. Fitzpatrick, J. R. Fowle, R. J. Kavlock, H. Kitano, B. A. Lidbury, A. R. Muotri, S. Q. Peng, D. Sakharov, T. Seidle, T. Trez, A. Tonevitsky, A. van de Stolpe, M. Whelan, and C. Willett, Lessons from Toxicology: developing a 21st-century paradigm for medical research, Environ. Health Perspect., 123, No. 11, A268-A272 (2015).
U. Marx, H. Walles, S. Hoffmann, G. Lindner, R. Horland, F. Sonntag, U. Klotzbach, D. Sakharov, A. Ronevitsky, and R. Lauster, Human-on-a-chip developments: a translational cutting-edge alternative to systemic safety assessment and efficiency evaluation of substances in laboratory animals and man? Altern. Lab. Anim., 40, No. 5, 235–257 (2012).
E. M. Materne, A. P. Ramme, A. P. Terrasso, M. Serra, P. M. Alves, C. Brito, D. A. Sakharov, A. G. Tonevitsky, R. Lauster, and U. Marx, A multi-organ chip co-culture of neurospheres and liver equivalents for long-term substance testing, J. Biotechnol., 205, 36–46 (2015).
E. M. Materne, A. G. Tonevitsky, and U. Marx, Chip-based liver equivalents for toxicity testing – organotypicalness versus cost-efficient high throughput, Lab Chip, 13, No. 18, 3481–3495 (2013).
A. Polini, L. Prodanov, N. S. Bhise, V. Manoharan, M. R. Dokmeci, and A. Khademhosseini, Organs-on-a-chip: a new tool for drug discovery, Experts Opin. Drug. Discov., 9, No. 4, 335–352 (2014).
N. V. Pul’kova, S. A. Tonevitskaya, V. M. Gerasimov, P. G. Rudakovskaya, A. G. Mazhuga, and D. A. Sakharov, Synthesis and optimization of methods for the production of magnetite nanoparticles with different sizes and morphology for biological application, Nanotechnologies in Russia, 10, Nos. 7–8, 570–575 (2015).
S. T. Selvan, T. T. Tan, D. K. Yi, and N. R. Jana, Functional and multifunctional nanoparticles for bioimaging and biosensing, Langmuir, 26, No. 14, 11631–11641 (2010).
N. V. Senyavina, E. V. Trushkin, A. L. Rusanov, V. A. Petrov, A. Yu. Shkurnikov, U. Marx, and D. A. Sakharov, Current technologies for in vitro testing of medicines: use of microbioreactors, Biotechnology in Russia, No. 1, 51–58 (2013).
I. Sergachev, A. Rusanova, E. Trushkin, D. Sakharov, U. Marx, and A. Tonevitsky, Fluorescent optical fiber sensors for cell viability monitoring, Analyst, 138, No. 14, 4066–4069 (2013).
I. Wagner, E. M. Materne, S. Brincker, U. Süssbier, Frädrich, M. Busek, F. Donntag, D. A. Sakharov, E. V. Trushkin, A. G. Tonevitsky, R. Lauster, and U. Marx, A dynamic multi-organchip for long-term cultivation and substance testing proven by 3D human liver and skin tissue co-culture, Lab Chip, 13, No. 18, 3538–3547 (2013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Byulleten’ Eksperimental’noi Biologii i Meditsiny, Vol. 161, No. 1, pp. 133–137, January, 2016
Rights and permissions
About this article
Cite this article
Sakharov, D.A., Rudakovskaya, P.G., Maltseva, D.V. et al. Modeling of Magnetite Nanoparticles Behavior under Conditions of Microcirculation and Analysis of In Vivo Toxicity. Bull Exp Biol Med 161, 116–119 (2016). https://doi.org/10.1007/s10517-016-3359-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-016-3359-5