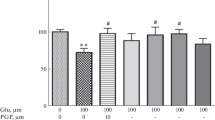
We studied cerebroprotective properties of neuropeptide cycloprolylglycine (1 mg/kg) administered intraperitoneally to rats with modeled incomplete global ischemia rats and neuroprotective properties for HT-22 cells under conditions of glutamate toxicity. It was shown that the neuropeptide administered during the postischemic period restored the neurological status of rats by preventing sensorimotor impairments in the limb-placing test and suppression of locomotor activity in the open field test. In in vitro experiments, cycloprolylglycine in concentrations of 10–5-10–8 M exhibited pronounced dose-dependent neuroprotective activity. The results attest to high cerebro- and neuroprotective potential of endogenous peptide cycloprolylglycine.
Similar content being viewed by others
References
T. A. Gudasheva, N. I. Vasilevich, R. U. Ostrovskaya, et al., Khim.-Farm. Zh., 30, No. 9, 12-17 (1996).
T. A. Gudasheva, M. A. Konstantinopol’skii, R. U. Ostrovskaya, and S. B. Seredenin, Bull. Exp. Biol. Med., 131, No. 5, 547-550 (2001).
T. A. Gudasheva, R. U. Ostrovskaya, F. V. Maksimova, et al., Khim.-Farm. Zh., 23, No. 3, 276-281 (1989).
T. A. Gudasheva, R. U. Ostrovskaya, S. D. Trofimov, et al., Bull. Exp. Biol. Med., 116, No. 10, 411-413 (1999).
I. V. Zarubina, Peptide Neuroprotection [in Russian], St. Petersburg (2009), pp. 126-185.
K. N. Kolyasnikova,T. A. Gudasheva, G. A. Nazarova, et al., Eksp. Klin. Farmakol., 75, No. 9, 3-6 (2012).
R. U. Ostrovskaya, E. A. Gudasheva, and T. A. Tsaplina, Bull. Exp. Biol. Med., 146, No. 9, 310-313 (2008).
A. H. Ahmed and R. E. Oswald, J. Med. Chem, 53, No. 5, 2197-2203 (2010).
J. Guan, S. Mathai, P. Harris, et al., Neuropharmacology, 53, No. 6, 749-762 (2007).
T. A. Gudasheva, S. S. Boyko, V. Kh. Akparov, et al., FEBS Lett., 391, No. 1-2, 149-152 (1996).
T. A. Gudasheva, S. S. Boyko, R.U. Ostrovskaya, et al., Eur. J. Drug Metab. Pharmacokinet., 22, No. 3, 245-252 (1997).
M. B. Hansen, S. E. Nielsen, and K. Berg, J. Immunol. Methods., 119, No. 2, 203-210 (1989).
J. Jolkkonen, K. Puurunen, S. Rantakomi, et al., Eur. J. Pharmacol., 400, Nos. 2-3, 211-219 (2000).
H. Jourdi, Y. T. Hsu, M. Zhou, et al., J. Neurosci., 29, No. 27, 8688-8697 (2009).
M. M. Muley, V. N. Thakare, R. R. Patil, et al., Life Sci., 93, No. 1, 51-57 (2013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Byulleten’ Eksperimental’noi Biologii i Meditsiny, Vol. 160, No. 11, pp. 600-603, November, 2015
Rights and permissions
About this article
Cite this article
Povarnina, P.Y., Kolyasnikova, K.N., Nikolaev, S.V. et al. Neuropeptide Cycloprolylglycine Exhibits Neuroprotective Activity after Systemic Administration to Rats with Modeled Incomplete Global Ischemia and in In Vitro Modeled Glutamate Neurotoxicity. Bull Exp Biol Med 160, 653–655 (2016). https://doi.org/10.1007/s10517-016-3241-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-016-3241-5