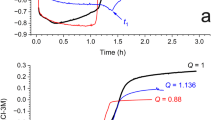
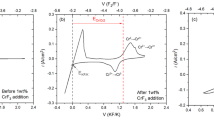
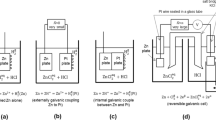
The present paper considers the physicochemical properties of valence electrons in the LiF–NaF–KF system within the framework of energy band theory. Here, the movement of the Fermi level in its band gap is equivalent to variations in the redox potential of the salt. When used to determine the limit level of the triple eutectic deoxidation, the electrochemical window identifi ed in the band gap may be suffi cient to suppress its corrosion activity in the liquid-salt reactor. However, this assumption, as well as the natural maintenance of this level in a mixture of three eutectic systems (LiF)0.99Li0.01, (NaF)0.97Na0.03, (KF)0.95K0.05, taken in the ratio of components (LiF)0.465(NaF)0.115(KF)0.42, requires experimental verification.
Similar content being viewed by others
References
L. I. Ponomarev, M. B. Seregin, A. P. Parshin, et al., “Selection of the fuel salt for a liquid salt reactor,” At. Energiya, 115, Iss. 1, 6–11 (2013).
R. Zvejskova, F. Lisy, and P. Soucek, “Electrochemical separation studies of selected actinides and lanthanides in molten fl uorides,” in: Proc. Intern. Conf. ATALANTE (Nimes), P1–67 (2004).
Y. Qin, Y. Zuo, and M. Shen, “Corrosion inhibition of 316L stainless steel in FLiNaK–CrF3/CrF2 redox buffering molten salt system,” J. Chin. Soc. Corr. Protect., 40, No. 2, 182–190 (2020).
A. Shimkevich, The Basics of Electrochemical Modifying of Liquid Dielectrics, Lambert Academic Publishing, Dusseldorf (2017).
Ch. Kittel, Introduction to Solid State Physics, Wiley, New York (1996).
P. Anderson, “Absence of diffusion in certain random lattices,” Phys. Rev., 109, No. 5, 1492–1505 (1958).
E. Chaleff, The Radiative Heat Transfer Properties of Molten Salts and Their Relevance to the Design of Advanced Reactors: PhD Degree Thesis, Ohio State University, Ohio (2016).
M. Bredig, Mixtures of Metals with Molten Salts, ORNL3391 (1963).
Y. Wasada-Tsutsui and H. Tatewaki, “Electronic band structure of crystalline NaF: ionization threshold and excited states related to lattice defects,” Surface Sci., 513, No. 1, 127–139 (2002).
Ch. Sommer, P. Kruger, and J. Pollmann, “Optical spectra of alkali-metal fl uorides,” Phys. Rev. B, 86, No. 15, 155212(1–7) (2012).
C. Bale, “The Li–Na (Lithium–Sodium) system,” Bull. Alloy Phase Diagrams, 10, No. 3, 265–268 (1989).
A. Shimkevich, “Introduction in the band theory of molten salts,” Phys. Lett. A, 383, No. 11, 1207–1213 (2019).
Sh. Guo, N. Shay, Ya. Wang, et al., “Measurement of europium (III)/europium (II) couple in fl uoride molten salt for redox control in a molten salt reactor concept,” J. Nucl. Mater., 496, 197–206 (2017).
R. Chesser, Sh. Guo, and J. Zhang, “Electrochemical behavior of dysprosium and lanthanum in molten LiF–NaF–KF (FLiNaK) salt,” Ann. Nucl. Energy, 120, 246–252 (2018).
A. G. Morachevskii, Thermodynamics of Molten Metals and Salts, Metallurgy, Moscow (1987).
K. Grjotheim, H. Ikeuchi, and J. Krogh-Moe, “The solution of alkaline earth metals in their molten halides,” Acta Chem. Scand., 24, No. 3, 985–990 (1970).
A. Shimkevich, “A true solubility versus the observed one for metal sodium in its molten chloride,” Chem. Phys., 540, 110975 (1–8) (2021).
M. Causo, G. Ciccotti, D. Montemayor, et al., “An adiabatic linear path integral approach for quantum time correlation functions: electronic transport in metal-molten salt solutions,” J. Phys. Chem. B, 109, No. 14, 6855–6865 (2005).
M. N. Ivanovskii, V. A. Morozov, N. N. Ponomarev-Stepnoy, and A. L. Shymkevich, “On the component phase transition of the first kind,” At. Energiya, 65, Iss. 5, 319–321 (1988).
D. Buceta, Y. Pineiro, C. Vazquez et al., “Metallic clusters: theoretical background, properties and synthesis in microemulsions,” Catalysts, 4, 356–374 (2014).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Atomnaya Énergiya, Vol. 133, No. 5–6, pp. 250–253, NovemberDecember, 2022.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shimkevich, A.L. Natural Suppression of Lif–Naf–Kf Corrosion Activity in a Liquid Salt Reactor. At Energy 133, 259–263 (2023). https://doi.org/10.1007/s10512-023-01005-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10512-023-01005-3