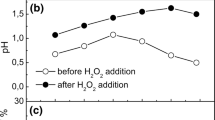
This article presents the results of experiments on electrochemical denitration of model solutions simulating aqueous raffinates of the Purex process and tail-water solutions after sorption extraction of plutonium. The experiments were performed with the use of a flow-through diaphragm electrochemical cell manufactured at the Vitold Bakhir Institute of Electrochemical Systems and Technologies. It is shown that the method of electrochemical reduction of nitric acid makes it possible to reduce the concentration of HNO3 from 3–7 to 0.5–1.4 mol/dm3 with using additional chemical reagents at room temperature. The specific amount of electricity spent on the decomposition of 1 g of HNO3 as well as the degree of migration of 137Cs and 152Eu from catholyte to anolyte in the process of electrochemical reduction, were determined. It is concluded that the method of electrochemical denitration shows promise in removing nitric acid from various types of liquid radioactive waste due to its low energy intensity, explosion safety, and the possibility of industrial implementation using home-grown process equipment.
Similar content being viewed by others
References
O. A. Ustinov, V. A. Kashcheev, and A. Yu. Shadrin, “Tritium in nitride fuel of fast reactors,” At. Energiya, 125, No. 4, 217–222 (2018).
L. Cecille and M. Lecomte, Radioactive Waste Management Series. Denitration of Radioactive Liquid Waste, L. Cecille and S. Halaszovich (eds.), Graham & Trotman, London (1986).
A. V. Ananiev, I. G. Tananaev, and V. P. Shilov, “Heterogeneous catalytic redox reactions in the chemistry and technology of the nuclear fuel cycle,” Adv. Chem., 47, No. 11, 1132–1155 (2005).
RF patent RU2593163C1, “Method for catalytic denitration of liquid radioactive waste,” subm. 2014, publ. July 27, 2016.
K. Fetter, Electrochemical Kinetics, Ya. M. Kolotyrkin (ed.), Khimiya, Moscow (1967).
R. K. Kvaratskheliya, Electrochemical Reduction of Oxygen Compounds of Nitrogen, Metsniereba, Tbilisi (1978).
A. M. Sukhotin (ed.), Handbook of Electrochemistry, Khimya, Tbilisi (1981).
V. M. Bakhir, Electrochemical Activation. Inventions, Hardware, Technology, VIVA-STAR, Moscow (2014).
P. G. Zelenin, V. V. Milyutin, V. M. Bakhir, and D. V. Adamovich, “Electrochemical oxidation of oxalate ions in aqueous solutions,” Radiokhimiya, 63, No. 4, 349–355 (2021).
P. G. Zelenin, V. V. Milyutin, A. F. Seliverstov, and V. M. Bakhir, “Electrochemical oxidation in aqueous solutions of organic complexing and surfactants,” Radiokhimiya, 64, No. 4, 382–388 (2022).
V. S. Koltunov and S. M. Baranov, ”Organic derivatives of hydrazine and hydroxylamine in the future technology for reprocessing irradiated nuclear fuel,” Radiokhimiya, 35, No. 6, 11–21 (1993).
K. N. Dvoeglazov, M. V. Logunov, L. N. Podrezova, et al., “Study of the kinetics of the interaction of diformylhydrazine with Pu(IV) ions in aqueous nitric acid solutions,” Abstracts of Radiochemistry-2012 Conference, Dimitrovgrad (2012), p. 122.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Atomnaya Énergiya, Vol. 133, No. 1, pp. 41–46, July, 2022.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Milyutin, V.V., Zelenin, P.G. Liquid Radwaste Denitration by Electrochemical Reduction of Nitric Acid. At Energy 133, 43–48 (2022). https://doi.org/10.1007/s10512-023-00970-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10512-023-00970-z