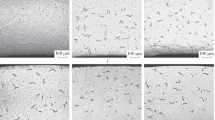
Local hydrogenation of zirconium fuel-element cladding, resulting in the formation of continuous hydrides, as a rule occurs when coolant or other hydrogen-containing compound finds its way beneath the fuel-element cladding irradiated in the core of a water moderated and cooled reactor. In this article, the set of processes occurring during hydrogenation of zirconium cladding is analyzed, and estimates are given for the parameters of these processes. The conditions and mechanism of formation of a continuous hydride over the thickness of zirconium cladding are established. It is shown that the duration of the nucleation of the hydride is determined primarily by the time to local destruction of the oxide film on the inner surface of the zirconium cladding of the fuel elements. The shortest possible time for continuous hydride to grow through the thickness of fuel-element cladding is determined.
Similar content being viewed by others
References
Waterside Corrosion of Zirconium Alloys in Nuclear Power Plants, IAEA-TECDOC-996, IAEA, Vienna (1998).
A. Couet, A. Motta, and R. Comstock, “Hydrogen pickup measurements in zirconium alloys: Relation to oxidation kinetics,” J. Nucl. Mater., 451, 1–13 (2014).
A. A. Shamkov, B. A. Kalin, V. M. Anan’yan, et al., “Plymouth solubility of hydrogen and zirconium alloys,” Vopr. At. Nauki Tekhn. Ser. Materialoved. Nov. Mater., No. 1(66), 366–370 (2006).
I. G. Lesina, V. B. Ivanov, and Ya. V. Sergeecheva, “Investigation of the interaction of hydrogen isotopes with the zirconium alloys E-110 and E-665 by radio luminography,” in: Abstr. All-Russ. Sci.-Techn. Conf. MAYAT-2014, Moscow (2014), pp. 34–35.
E. Yu. Afanas’eva, I. A. Evdokimov, V. V. Likhanskii, et al., “Modeling of hydride destruction of fuel elements in water cooled reactors,” At. Energ., 95, No. 4, 275–283 (2003).
Review of Fuel Failures in Water Cooled Reactor, No. NFT-2.1, IAEA Vienna (2010).
M. Yamamoto, S. Naito, M. Mabuchi, and T. Hashino, “Adsorption potential of hydrogen atom on zirconium,” J. Phys. Chem., 96, 3409–3412 (1992).
M. Yamamoto, S. Naito, M. Mabuchi, and T. Hashino, “Kinetics of oxygen adsorption by alpha-zirconium,” J. Phys. Chem., 93, 5203–5209 (1989).
A. Sawatzky, “The diffusion and solubility of hydrogen in the alpha phase of Zircaloy-2,” J. Nucl. Mater., 2, No. 1, 62–68 (1960).
M. Mallett and W. Albretcht, “Low-pressure solubility and diffusion of hydrogen in zirconium,” J. Electrochem. Soc., 104, No. 3, 142–146 (1957).
J. Kearns, “Diffusion coefficient of hydrogen in alpha zirconium Zircaloy-2 and Zircaloy-4,” J. Nucl. Mater., 43, 330–338 (1972).
E. Zuzek, “On equilibrium in the Zr–H system,” Surf. Coat. Technol., 28, No. 3–4, 323–338 (1986).
G. Majer, W. Renz, and R. Barnes, “The mechanism of hydrogen diffusion in zirconium dihydrides,” J. Phys., 6, 2935–2942 (1994).
E. Zuzek and J. Abriata, “The H–Zr (hydrogen-zirconium) system,” Bul. Alloy Phase Diag., 11, No. 4, 385–395 (1990).
D. Khatamian and W. Manchester, “An ion beam study of hydrogen diffusion in oxides of Zr and Zr–Nb (2.5 wt%). I. Diffusion parameters for dense oxide,” J. Nucl. Mater., 166, 300–306 (1989).
D. Khatamian, “Hydrogen diffusion in oxides formed on surfaces of zirconium alloys,” J. Alloys Comp., 253–254, 471–474 (1997).
D. Khatamian, “Diffusion of hydrogen in single crystals of monoclinic ZrO2 and yttrium stabilized cubic zirconia,” Defect Diff. For., 297–301, 631–640 (2010).
B. Lim, H. Hong, and K. Lee, “A study on the effects of dissolved hydrogen on zirconium alloys corrosion,” J. Nucl. Mater., 444, 349–355 (2014).
A. A. Shmakov, E. A. Smirnov, and Kh. Brukhertzoifer, “Distribution and diffuion of hydrogen in oxidized alloys based on zirconium,” At. Energ., 85, No. 3, 253–255 (1998).
J. Austin, T. Elleman, and K. Verghese, “Tritium diffusion in zircaloy-2 in the temperature range –78 to 204°C,” J. Nucl. Mater., 51, 321–329 (1974).
Y. Hatano, R. Hitaka, M. Sugisaki, and M. Hayashi, “Transport mechanism of hydrogen through oxide fi lm formed on Zircaloy-4,” J. Rad. Nucl. Chem., 239, No. 3, 445–448 (1999).
W. Kunz, H. Münzel, and U. Kunz, “Tritium release from Zircaloy-2: Dependence on temperature, surface conditions and composition of surrounding medium,” J. Nucl. Mater., 136, 6–15 (1985).
I. Takagi, K. Une, S. Miyamura, and T. Kobayashi, “Deuterium diffusion in steam-corroded oxide layer of zirconium alloys,” J. Nucl. Mater., 419, 339–346 (2011).
N. McIntyre, R. Davidson, C. Weisener, et al., “SIMS studies of hydrogen diffusion through oxides on Zr–Nb alloy,” Surf. Interf. Anal., 17, 757–763 (1991).
V. A. Markelov, Improvements of the Composition and Structure of Zirconium Alloys in Order to Ensure the Serviceability of Fuel Elements, Fuel Assemblies, and Pressure Piping in the Cores of Water Cooled Reactors with Extended Service Life and Increased Fuel Burnup: Auth. Abstr. Dissert. Doct. Techn. Sci., Moscow (2010).
Author information
Authors and Affiliations
Additional information
Translated from Atomnaya Énergiya, Vol. 123, No. 6, pp. 321–329, December, 2017.
Rights and permissions
About this article
Cite this article
Novikov, V.V., Khomyakov, O.V. & Devyatko, Y.N. Mechanism and Conditions for Continuous Hydride Layer Formation in Zirconium Cladding. At Energy 123, 389–398 (2018). https://doi.org/10.1007/s10512-018-0358-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10512-018-0358-9