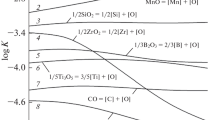
It is confirmed experimentally that water is soluble in sodium molybdate metal, and the solubility at 800°C is 0.018 wt.%. On the basis of this phenomenon, a technology is proposed for dewatering zirconium articles, which are subjected to heating in sodium molybdate, by introducing 1–4 wt.% NiMoO4. As a result, the hydrogen content in the article decreases from 10–2 to 0.4·10–3%. It is confirmed experimentally that metallic nickel, with which dissolved water reacts with hydrogen being released, introduced into the melt reduces U3O8 to UO2 in sodium molybdate melt.
Similar content being viewed by others
References
V. P. Kochergin, Z. A. Shevrina, and V. G. Berner, “Corrosion of iron in melts containing alkali-metal phosphates and fluorides,” Izv. Vyssh. Uchebn. Zaved. Khim. Khim. Tekhnol., 10, No. 3. 288–292 (1967).
V. P. Kochergin, L. G. Khaibullina, and O. G. Potapova, “Dissolution of iron in fused zinc chloride and chlorides of alkali and alkaline-earth metals,” Zh. Neorg. Khim., 1, No. 11, 2617–2622 (1956).
L. E. Ivanovskii and V. N. Nekrasov, Gases and Ionic Melts, Nauka, Moscow (1979).
S. I. Poppel, A. I. Sotnikov, and V. N. Barannikkov, Theory of Metallurgical Processes, Metallurgiya, Moscow (1979).
N. N. Fedotova, Thermodynamics of Solutions of Gases, Water Vapor and Ammonia in Fused Alkali-Metal Nitrates: Auth. Abstr. Dissert. Cand. Chem. Sci., Kabardino-Balkaria State University, Nalchik (2003).
T. G. Fedorov, Spectral Isotopic Analysis of Hydrogen and Determination of the Hydrogen Concentration in Metals, Atomizdat, Moscow (1980).
O. A. Ustinov, L. P. Sukhanov, and O. N. Pogorelko, “Reductive recrystallization of uranium oxide in molybdate melts,” At. Énerg., 102, No. 5, 290–292 (2007).
V. K. Markov, Methods for Determining Uranium, Atomizdat, Moscow (1964).
Author information
Authors and Affiliations
Additional information
* Deceased.
Translated from Atomnaya Énergiya, Vol. 117, No. 1, pp. 30–33, July, 2014.
Rights and permissions
About this article
Cite this article
Ustinov, O.A., Matyushin*, E.A. & Fedorov*, T.G. Water in Molybdate Melts and Reactions with its Participation. At Energy 117, 36–39 (2014). https://doi.org/10.1007/s10512-014-9884-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10512-014-9884-2