Abstract
The effect of stocking density on the rearing performance of pikeperch juveniles was studied. Three separate experiments (I, II and III) were conducted with fish of an initial average body weight of 1.3, 6.7 and 19.2 g, respectively. Each experimental design consisted of three treatments (three replicates) with different initial stocking densities (low, medium and high). Experiments lasted 28 days in a recirculating aquaculture system (21°C, 24L:0D) with an initial stocking density of 0.78, 1.04 and 1.30 kg m−3 in experiment I, 2.68, 4.02 and 5.36 kg m−3 in experiment II, and 3.84, 7.68 and 11.52 kg m−3 in experiment III. The results of our study showed that in experiment I, the use of a stocking density of 1.04 kg m−3 resulted in the highest body weight and survival, as well as the lowest feed conversion ratio and cannibalism. In experiments II and III, the pikeperch growth rate decreased, and their feed conversion ratio increased gradually with increasing stocking density. Our study demonstrated that based on growth parameters, densities of 1.04, 2.68 and 3.84 kg m−3 can be used for pikeperch with an initial body weight of 1.3, 6.7 and 19.2 g, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intensive aquaculture in recirculating aquaculture systems (RAS) has advantages (low water usage, constant water quality and low environmental impact) and disadvantages (high initial costs and high operating costs) in comparison to traditional pond systems (Martins et al. 2010; Nagy et al. 2022). Pikeperch Sander lucioperca (L.) is one of the most promising species cultured in RAS (Policar et al. 2019). In terms of diversification, pikeperch is an attractive alternative to other inland species reared in aquaculture (Dalsgaard et al. 2013; Baekelandt et al. 2018; Nagy et al. 2022). Due to its high-quality meat, high market and fast growth, the species has been considered for aquaculture intensification primarily in Western and Central Europe (Policar et al. 2019). Nevertheless, pikeperch culture remains limited mainly because of low survival rates, cannibalism and deformities in larval stages (Szkudlarek and Zakęś 2007; Dalsgaard et al. 2013; Colchen et al. 2020). Previous studies have indicated that juvenile pikeperch can be cultured successfully in RAS. Many studies have evaluated the influences of temperature (Rónyai and Csengeri 2008; Wang et al. 2009), light intensity (Luchiari et al. 2006; Kozłowski et al. 2010), water quality (Steenfeldt et al. 2010; Dalsgaard et al. 2013), feeding frequency (Zakęś et al. 2006; Wang et al. 2009; Pěnka et al. 2023), feed pellet size (Mattila and Koskela 2017; Kozłowski et al. 2021) and stocking density (Szkudlarek 2002; Molnár et al. 2004; Steenfeldt et al. 2010) on the growth of pikeperch.
Stocking density is an important factor determining production efficiency in intensive aquaculture systems (Riche et al. 2013), influencing growth, water quality and fish welfare (Salas-Leiton et al. 2010; Oppedal et al. 2011; Manley et al. 2014; Yang et al. 2020). Depending on fish species, extreme low or high densities can have either positive (Liu et al. 2019; Ni et al. 2016; Yang et al. 2020) or negative effects on fish welfare and growth (Adams et al. 2007; Jørgensen et al. 1993; Millán-Cubillo et al. 2016). The complex relationship between fish welfare and stocking density means that the determination of optimal stocking densities is difficult. One species’ reactions to stocking density can differ significantly depending on fish size and the system in which they are farmed (Ellis et al. 2002). Thus, stocking density is a key factor influencing the health of fish in commercial production, and it is of particular concern regarding fish welfare (Li et al. 2021). In the case of pikeperch, information on the stocking density is limited to small (< 1 g) fish (Szkudlarek 2002; Molnár et al. 2004). For fish bigger than 1 g, most of the literature data is based only on the report of Steenfeldt et al. (2010), who demonstrated that pikeperch weighing from 10 to 50 g should be kept at densities lower than 15–30 kg m−3, while fish weighing up to 2 kg can be kept at densities of approximately 30–60 kg m−3. Such densities do not increase stress levels, growth rates or the feed conversion ratio. Currently, there is no information on the effects of stocking density on production performance of pikeperch sizing 1 to 10 g.
The aim of the present study was to assess the influence of stocking density on the growth, survival and cannibalism of juvenile pikeperch in three-size groups fed commercial food and reared under controlled conditions. Three separate experiments were conducted in controlled conditions with fish of different initial mean body weights (1.3, 6.7 and 19.2 g).
Materials and methods
Rearing conditions and experiment design
The study was conducted at the Department of Sturgeon Breeding in Pieczarki of the National Inland Fisheries Research Institute in Olsztyn, Poland. Three separate experiments (I, II and III) were performed using different fish sizes. In experiment I (fish age 67 days post-hatch [DPH]), the mean pikeperch body weight was 1.3 ± 0.1 g (mean values ± standard deviation) with a mean body length of 5.4 ± 0.1 cm. In experiment II, pikeperch (100 DPH) weighed 6.7 ± 0.2 g with a mean body length of 8.1 ± 0.2 cm, while in experiment III (130 DPH), they weighed 19.2 ± 0.2 g and had a body length of 11.7 ± 0.2 cm. Each experiment (I, II and III) was performed in three experimental groups and three replicates and each lasted 28 days. The fish were reared in 9 tanks with working volumes of 1.0 m3 each (1.2 m × 1.2 m × 0.7 m). All experiments were performed in the same recirculating system one after the other. The tanks were part of a RAS equipped with an oxygen generator (OGP 4, Atlas Copco, Netherlands), micro sieve (Hydrotech filter, Veolia, Sweden) and a biofilter with a volume of 3.2 m3 (SDK CN 3.2, SDK, Poland) with plastic filling (Light Bioelementer RK Plast A/S, Denmark) of a total volume of 1.5 m3. The filter thickness was 0.93 g cm−3, and its surface area proper was 750 m2 m−3. Throughout the experiments, the photoperiod was 24-h light at an intensity of 100 lux measured above water surfaces.
Each experiment consisted of three treatments with different (low, L; medium, M; and high, H) stocking densities:
-
Experiment I: LI, stocking density 600 inds. m−3 (0.78 kg m−3); MI, stocking density 800 inds. m−3 (1.04 kg m−3); HI, stocking density 1000 inds. m−3 (1.30 kg m−3)
-
Experiment II: LII, stocking density 400 inds. m−3 (2.68 kg m−3); MII, stocking density 600 inds. m−3 (4.02 kg m−3); HII, stocking density 800 inds. m−3 (5.36 kg m−3)
-
Experiment III: LIII, stocking density 200 inds. m−3 (3.84 kg m−3); MIII, stocking density 400 inds. m−3 (7.68 kg m−3); HIII, stocking density 600 inds. m−3 (11.52 kg m−3)
The daily feed rations and feed pellet sizes for the different experiments were determined based on previous studies (Kozłowski et al. 2021). The fish were fed commercial sinking feed manufactured by Aller Aqua (Denmark). Thalassa Ex GR 0.9–1.6 mm was used in experiment I, Thalassa Ex GR 1.3–2.0 mm in experiment II and Thalassa Ex GR 1.6–2.4 mm in experiment III. All of the feeds contained 54% protein, 15% fat and 8.5% carbohydrates. Feed was delivered by automatic band feeders (Fischtechnik GmbH, Germany) for 18 h day−1.
Physical and chemical analyses of water
Oxygen content and pH were measured with a CyberScan PCD 5500 (Eutech Instruments, USA). Total ammonia nitrogen (TAN = NH4+-N + NH3-N) and nitrite (NO2-N) concentration were determined with an Aquamate Plus UV-Vis spectrophotometer (Thermo Scientific, England). Water quality parameters were measured at least three times weekly, and water temperature was measured daily. The average water temperature during the experiments was 21.0 ± 0.8 °C. Water oxygen concentration at the tank outflows did not decrease below 7.0 mg O2 l−1, and pH ranged from 7.6 to 7.8. The maximum concentrations of ammonia nitrogen and nitrite did not exceed 0.27 mg l−1 or 0.36 mg l−1, respectively. All water quality parameters did not differ among the experiments and in between treatments (P > 0.05). The water flow in the experimental tanks was 12 l min−1.
Data collection and statistical analyses
The biomass and number of fish in each tank were determined at the beginning and the end of each of the three experiments. The biomass was estimated by bulk-weighing the whole fish population from each tank. Additionally, on days 1, 7, 14, 21 and 28 of the experiments, individual measurements of body weight (BW) and total length (TL) were performed for 50 specimens from each tank to determine growth parameters and weekly feed rations. Before the measurements, fish were anaesthetized in a solution of Propiscin (active ingredient—etomidate) at a concentration of 0.8 ml l−1. Fish mortality was monitored and recorded daily. These data were used to calculate the following parameters:
-
Specific growth rate: SGR (% d−1) = 100 × (ln BW2 − ln BW1) × T−1
-
Condition factor: K = 100 × BW × BL−3
-
Feed conversion ratio: FCR = TFC × (FB − IB)−1
-
Daily feed intake: DFI (%BW d−1) = FC × 100 [(IB + FB) × 2−1)] −1 × T−1
-
Coefficient of variation for body weight: CV (%) = 100 × SD × BW−1
-
Survival: S (%) = 100 (FN IN−1)
-
Cannibalism: C (%) = 100 [IN − (FN + DI)] IN−1
-
Biomass gain: BG (%) = 100 × (FB − IB) × IB−1
where BW1 is the initial body weight (g), BW2 is the final body weight (g), BW is the body weight (g), T is the rearing period (days), BL is the body length (cm), SD is the body weight standard deviation, IB is the initial fish biomass (g), FB is the final fish biomass (g), IN is the initial number of fish (individuals), FN is the final number of fish (individuals), TFC is the total feed consumption (g) and DI is the number of dead specimens during rearing (individuals).
Mean values and standard deviations (SD) are presented. The normality of parameter distribution was tested by the Shapiro-Wilk test and, to confirm the homogeneity of variance, Levene’s test. The data expressed in percentages were arcsin transformed before the statistical analysis. Data were compared using one-way ANOVA. The significance of differences was estimated using a post hoc HSD Tukey test (P < 0.05). Analyses were performed using STATISTICA 12 PL software (StatSoft, Poland).
Results
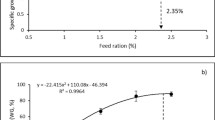
In experiment I, fish in the medium stocking density presented significantly higher final body weight than in the low and high stocking densities (P < 0.05). A similar trend was observed for FCR, with fish at medium density presenting significantly lower FCR than in low stocking density (Table 1; P < 0.05). Survival and cannibalism were also significantly better at 1.04 kg m−3 densities (P < 0.05) when comparing with the other stocking densities tested, demonstrating that fish performed better when reared at medium stocking density. In experiment II, pikeperch of initial body weight of 6.7 g performed (final body weight, SGR and FCR) significantly better and presented lower DFI at low density when compared with higher density (Table 2; P < 0.05). In experiment III, fish final body weight and SGR were significantly higher at low stocking density when compared with the medium and high stocking density (Table 3; P < 0.05). A similar trend was observed for FCR, with fish at low densities presenting significantly lower FCR than the other treatments (P < 0.05).
Discussion
Stocking density is an environmental stress factor that influences fish growth (Liu et al. 2017). In the present study, low initial stocking densities were applied to the three size groups (0.78–11.52 kg−3) because of the better understanding of how optimal growth parameters can be achieved. In experiment I, the final body weight and SGR value in LI treatment at a final stocking density of 2.72 kg m−3 (initial stocking density 0.78 kg-3) were lower than those in MI treatment (final stocking density 4.27 kg−3), which indicated that lower stocking density had an adverse effect on the growth of pikeperch of an initial body weight (BWi) of 1.3 g. Other studies have also shown that low stocking density can be stressful because of social interactions among fish (Manley et al. 2014; Millán-Cubillo et al. 2016). In this study, lower body weight at low stocking densities was associated with higher FCR and cannibalism. Pikeperch sizing 1.3 to 5.0 g may require a minimum stocking density to achieve better performance. Low densities could be detrimental for social and/or territorial fish species as it could trigger competition for food, increased appetite (Potthoff and Christman 2006) and stressful conditions (Strand et al. 2007).
In experiments II and III, the fish performance was negatively impacted with increasing density. Similar results were also noted in studies of Amur sturgeon Acipenser schrenckii (von Brandt) (Yang et al. 2011), turbot Scophthalmus maximus (L.) (Liu et al. 2017), lenok Brachymystax lenok (Pallas) (Liu et al. 2019), Atlantic salmon Salmo salar (L.) (Oppedal et al. 2011) and pompano Trachinotus ovatus (L.) (Yang et al. 2020). The results of the present study showed an increase in daily feed intake with increasing stocking density. This was not reflected in the feed conversion ratio which increased with increasing stocking density. The limited growth of pikeperch at high stocking density was the result of crowding stress which triggered a rising demand for energy to activate the physiological responses to cope with stress, and led to a reduction in the available energy for growth (Yang et al. 2020). Moreover, at higher stocking densities, fish compete for food and expend more energy on movement, resulting in lower body weight gain (Yang et al. 2011).
It is well known that fish survival is negatively impacted with increase in stocking density (Hitzfelder et al. 2006). Many studies have shown that survival often decreases with increasing stocking density (Rahman et al. 2005; Hitzfelder et al. 2006; Rowland et al. 2006). In our study, differences in survival of the small pikeperch with body weight of 1.3 g resulted from higher cannibalism at the high (HI) and low (LI) stocking densities. Cannibalism also occurred in medium-sized fish of 6.7 g (BWi) but at a low level (1–2%) compared with the small fish size. No cannibalism was observed within the larger fish size used in this experiment, with survival > 99% for the three stocking densities tested. Kozłowski et al. (2021) reported similar results when rearing pikeperch of a similar size. The results of this and previous studies (Kozłowski et al. 2021) suggest that cannibalism of pikeperch with a body length over 11 cm is stopped.
The stocking densities used in this study affected the growth of juvenile pikeperch. Another study showed no difference in growth parameters for pikeperch of 0.65 g (BWi) and stocking density 0.99–2.31 kg m−3, possibly due to high cannibalism (37–40%) (Szkudlarek 2002). Similar results were obtained by Molnár et al. (2004) for pikeperch of 0.91 g (BWi) and stocking density of 1.25–2.08 kg m−3. For larger pikeperch sizing 91 g, high stocking density (15–30 kg m−3) did not negatively impacted growth and feed utilization (Baekelandt et al. 2018).
Stocking density is regarded as a priority topic in aquaculture research because of its influences on the welfare of cultured fish and the need for formulating further recommendations on this issue. The growth of small pikeperch (1.3 g) was slightly but significantly higher at medium stocking densities (1.04 kg m−3) than at low and high stocking densities (0.78 and 1.04 kg m−3, respectively). For the medium-sized (6.7 g) and large-sized (19.2 g) fish, growth was slightly but significantly higher at lower densities (2.68 and 3.84 kg m−3, respectively) than at densities ranging from 4.02 to 11.52 kg m−3. The results of the present study indicated that, under the same experimental conditions, pikeperch of 1.3, 6.7 and 19.2 g (BWi) achieved faster growth at medium to low stocking density of 1.04, 2.68 and 3.84 kg m−3, respectively. However, low stocking densities might not be economically viable. Therefore, optimal stocking density should be assessed based on both fish performance and economic feasibility. In our study, the condition factor, which is a good indicator of health status, was similar in all the experiments and at all the stocking densities. This suggests that juvenile pikeperch were properly fed, although FCR < 1 may indicate an underestimation of the feed dose. Further studies should focus on the influence of stocking density on physiological, immune, body composition and water quality parameters.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Adams CE, Turnbull JF, Bell A, Bron JE, Huntingford FA (2007) Multiple determinants of welfare in farmed fish: Stocking density, disturbance, and aggression in Atlantic salmon (Salmo salar). Can J Fish Aquat Sci 64(2):336–344. https://doi.org/10.1139/f07-018
Baekelandt S, Redivo B, Mandiki SNM, Bournonville T, Houndji A, Bernard B, El Kertaoui N, Schmitz M, Fontaine P, Gardeur J-N, Ledoré Y, Kestemont P (2018) Multifactorial analyses revealed optimal aquaculture modalities improving husbandry fitness without clear effect on stress and immune status of pikeperch Sander lucioperca. Gen Comp Endocrinol 258:194–204. https://doi.org/10.1016/j.ygcen.2017.08.010
Colchen T, Gisbert E, Krauss D, Ledoré Y, Pasquet A, Fontaine P (2020) Improving pikeperch larviculture by combining environmental, feeding and populational factors. Aquac Rep 17:100337. https://doi.org/10.1016/j.aqrep.2020.100337
Dalsgaard J, Lund I, Thorarinsdottir R, Drengstig A, Arvonen K, Pedersen PB (2013) Farming different species in RAS in Nordic countries: current status and future perspectives. Aquac Eng 53:2–13. https://doi.org/10.1016/j.aquaeng.2012.11.008
Ellis T, North B, Scott AP, Bromage NR, Porter M, Gadd D (2002) The relationships between stocking density and welfare in farmed rainbow trout. J Fish Biol 61(3):493–531. https://doi.org/10.1111/j.1095-8649.2002.tb00893.x
Hitzfelder GM, Holt GJ, Fox JM, McKee DA (2006) The effect of rearing density on growth and survival of cobia, Rachycentron canadum, larvae in a closed recirculating aquaculture system. J World Aquacult Soc 37(2):204–209. https://doi.org/10.1111/j.1749-7345.2006.00028.x
Jørgensen EH, Christianssen JS, Jobling M (1993) Effects of stocking density on food intake, growth performance and oxygen consumption in Arctic charr (Salvelinus alpinus L). Aquaculture 110(2):191–204. https://doi.org/10.1016/0044-8486(93)90272-Z
Kozłowski M, Piotrowska I, Szczepkowska B (2021) Effect of feed pellet size and tank water level on growth performance in juvenile pikeperch, Sander lucioperca (L.), reared in a recirculating system. Fish Aquat Life 29(2):88–99. https://doi.org/10.2478/aopf-2021-0011
Kozłowski M, Zakęś Z, Szczepkowski M, Wunderlich K, Piotrowska I, Szczepkowska B (2010) Impact of light intensity on the results of rearing juvenile pikeperch, Sander lucioperca (L.), in recirculating aquaculture systems. Arch Pol Fish 18(2):77–84. https://doi.org/10.2478/v10086-010-0009-9
Li L, Shen Y, Yang W, Xu X, Li L (2021) Effect of different stocking densities on fish growth performance: a meta-analysis. Aquaculture 544:737152. https://doi.org/10.1016/j.aquaculture.2021.737152
Liu B, Jia R, Zhao K, Wang G, Lei J, Huang B (2017) Stocking density effects on growth and stress response of juvenile turbot (Scophthalmus maximus) reared in land-based recirculating aquaculture system. Acta Oceanol Sin 36(10):31–38. https://doi.org/10.1007/s13131-017-0976-4
Liu Y, Liu H, Wua W, Yina J, Mouc Z, Hao F (2019) Effects of stocking density on growth performance and metabolism of juvenile Lenok (Brachymystax lenok). Aquaculture 504:107–113. https://doi.org/10.1016/j.aquaculture.2019.01.058
Luchiari AC, de Morais Freire FA, Koskela J, Pirhonen J (2006) Light intensity preference of juvenile pikeperch Sander lucioperca (L.). Aquac Res 37(15):1572–1577. https://doi.org/10.1111/j.1365-2109.2006.01599.x
Manley CB, Rakocinski CF, Lee PG, Blaylock RB (2014) Stocking density effects on aggressive and cannibalistic behaviors in larval hatchery-reared spotted seatrout, Cynoscion nebulosus. Aquaculture 420-421:89–94. https://doi.org/10.1016/j.aquaculture.2013.10.040
Mattila J, Koskela J (2017) Effect of feed pellet size on production parameters of pike-perch (Sander lucioperca). Aquac Res 49(1):586–590. https://doi.org/10.1111/are.13443
Martins CIM, Eding EH, Verdegem MCJ, Heinsbroek LTN, Schneider O, Blancheton JP, Roque d’Orbcastel E, Verreth JAJ (2010) New developments in recirculating aquaculture systems in Europe: a perspective on environmental sustainability. Aquac Eng 43(3):83–93. https://doi.org/10.1016/j.aquaeng.2010.09.002
Millán-Cubillo AF, Martos-Sitcha JA, Ruiz-Jarabo I, Cárdenas S, Mancera JM (2016) Low stocking density negatively affects growth, metabolism and stress pathways in juvenile specimens of meagre (Argyrosomus regius, Asso 1801). Aquaculture 451:87–92. https://doi.org/10.1016/j.aquaculture.2015.08.034
Molnár T, Hancz C, Bódis M, Müller T, Bercsényi M, Horn P (2004) The effect of initial stocking density on growth and survival of pike-perch fingerlings reared under intensive conditions. Aquac Int 12(2):181–189. https://doi.org/10.1023/B:AQUI.0000032079.62056.8c
Nagy Z, Ardó L, Demény F, Gál D, Sándor ZJ, Ljubobratović U (2022) Case study on the aptness of in-pond raceways for pikeperch, Sander lucioperca, grow-out. Aquac Rep 27:101356. https://doi.org/10.1016/j.aqrep.2022.101356
Ni M, Wen H, Li J, Chi M, Bu Y, Ren Y, Zhang M, Song M, Ding H (2016) Effects of stocking density on mortality, growth and physiology of juvenile Amur sturgeon (Acipenser schrenckii). Aquac Res 47(5):1596–1604. https://doi.org/10.1111/are.12620
Oppedal F, Vågseth T, Dempster T, Juell J, Johansson D (2011) Fluctuating sea-cage environments modify the effects of stocking densities on the production and welfare of Atlantic salmon (Salmo salar L.). Aquaculture 315(3-4):361–368. https://doi.org/10.1016/j.aquaculture.2011.02.037
Pěnka T, Malinovskyi O, Imentai A, Kolářová J, Kuĉera V, Policar T (2023) Evaluation of different feeding frequencies in RAS-based juvenile pikeperch (Sander lucioperca) aquaculture. Aquaculture 562:738815. https://doi.org/10.1016/j.aquaculture.2022.738815
Policar T, Schaefer FJ, Panana E, Meyer S, Teerlinck S, Toner D, Żarski D (2019) Recent progress in European percid fish culture production technology-Tackling bottlenecks. Aquac Int 27(5):1151–1174. https://doi.org/10.1007/s10499-019-00433-y
Potthoff MT, Christman MC (2006) Growth depensation and group behaviour in juvenile hybrid striped bass Morone chrysops x Morone saxatilis: effects of group membership, feeding method, ration size and size disparity. J Fish Biol 69(3):828–845. https://doi.org/10.1111/j.1095-8649.2006.01162.x
Rahman MA, Mazid MA, Rahman MR, Khan MN, Hossain MA, Hussain MG (2005) Effect of stocking density on survival and growth of critically endangered mahseer, Tor putitora (Hamilton) in nursery ponds. Aquaculture 249(1-4):275–284. https://doi.org/10.1016/j.aquaculture.2005.04.040
Riche MA, Weirich CR, Wills PS, Baptiste RM (2013) Stocking density effects on production characteristics and body composition of market size cobia, Rachycentron canadum, reared in recirculating aquaculture systems. J World Aquacult Soc 44(2):259–266. https://doi.org/10.1111/jwas.12023
Rónyai A, Csengeri I (2008) Effect of feeding regime and temperature on ongrowing results of pikeperch (Sander lucioperca L.). Aquac Res 39(8):820–827. https://doi.org/10.1111/j.1365-2109.2008.01935.x
Rowland SJ, Mifsud C, Nixon M, Boyd P (2006) Effects of stocking density on the performance of the Australian freshwater silver perch (Bidyanus bidyanus) in cages. Aquaculture 253(1-4):301–308. https://doi.org/10.1016/j.aquaculture.2005.04.049
Salas-Leiton E, Anguis V, Martín-Antonio B, Crespo D, Planas JV, Infante C, Cañavate JP, Manchado M (2010) Effects of stocking density and feed ration on growth and gene expression in the Senegalese sole (Solea senegalensis): potential effects on the immune response. Fish Shellfish Immunol 28(2):296–302. https://doi.org/10.1016/j.fsi.2009.11.006
Steenfeldt SJ, Vestergaard M, Overton JL, Lund I, Paulsen H, Larsen VJ, Henriksen NH (2010) Videreudvikling af intensivt opdræt af sandart i Danmark. DTU Aqua. DTU Aqua-rapport Nr. pp 228–2010.
Strand A, Alanärä A, Magnhagen C (2007) Effect of group size on feed intake, growth and feed efficiency of juvenile perch. J Fish Biol 71(2):615–619. https://doi.org/10.1111/j.1095-8649.2007.01497.x
Szkudlarek M, Zakęś (2002) The effect of stock density on the effectiveness of rearing pikeperch Sander lucioperca L. summer fry. Arch Pol Fish 10(1):115–119
Szkudlarek M, Zakęś (2007) Effect of stocking density on survival and growth performance of pikeperch, Sander lucioperca (L.), larvae under controlled conditions. Aquac Int 15(1):67–81. https://doi.org/10.1007/s10499-006-9069-7
Wang N, Xu X, Kestemont P (2009) Effect of temperature and feeding frequency on growth performances, feed efficiency and body composition of pikeperch juveniles (Sander lucioperca). Aquaculture 289(1-2):70–73. https://doi.org/10.1016/j.aquaculture.2009.01.002
Yang DG, Zhu YJ, Luo YP, Zhao JH, Chen JW (2011) Effect of stocking density on growth performance of juvenile Amur Sturgeon (Acipenser schrenckii). J Appl Ichthyol 27(2):541–544. https://doi.org/10.1111/j.1439-0426.2011.01705.x
Yang Q, Guo L, Liu B-S, Guo H-Y, Zhu K-C, Zhang N, Jiang S-G, Zhang D-C (2020) Effects of stocking density on the growth performance, serum biochemistry, muscle composition and HSP70 gene expression of juvenile golden pompano Trachinotus ovatus (Linnaeus, 1758). Aquaculture 518:734841. https://doi.org/10.1016/j.aquaculture.2019.734841
Zakęś Z, Kowalska A, Czerniak S, Demska-Zakęś K (2006) Effect of feeding frequency on growth and size variation in juvenile pikeperch, Sander lucioperca (L.). Czech J Anim Sci 51(2):85–91. https://doi.org/10.17221/3914-CJAS
Acknowledgements
The study was conducted within the framework of the statutory research program of the National Inland Fisheries Research Institute in Olsztyn (No. Z-013 and Z-016). Publication cost was co-financed by the fund of the National Inland Fisheries Research Institute in Olsztyn.
Author information
Authors and Affiliations
Contributions
Conceptualization: MK; methodology: MK, IP; formal analysis and investigation: MK, IP; writing—original draft preparation: MK; writing—review and editing: MK, IP; resources: MK, IP; supervision: MK. All the authors approved the final draft.
Corresponding author
Ethics declarations
Ethical approval
The study was in compliance with Polish animal welfare regulations and approved by the Local Ethics Committee for Animal Experimentation of the National Inland Fisheries Research Institute in Olsztyn, Poland.
Conflict of interest
The authors declare no competing interests.
Additional information
Handling editor: Gavin Burnell
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kozłowski, M., Piotrowska, I. Effect of stocking density on growth, survival and cannibalism of juvenile pikeperch, Sander lucioperca (L.), in a recirculating aquaculture system. Aquacult Int 32, 3587–3595 (2024). https://doi.org/10.1007/s10499-023-01339-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01339-6