Abstract
The present study investigated the effect of Chlorella vulgaris on growth performance, feed consumption, body composition, and immune response of white shrimp (Litopenaeus vannamei) against Vibrio parahaemolyticus infection. Shrimps (4.00 ± 0.04 g) were divided into five groups (3 replicates/20 each) in 15 hapa (1 m3) and cultured for 56 days with five C. vulgaris experimented diets at five different concentrations (0, 15, 20, 25, and 30 g/kg feed). Growth performance, feed utilization, biomass, and body composition (protein, lipid, and ash) were analyzed and revealed significant increase (P < 0.05) in shrimp fed the high C. vulgaris diet. Additionally, the survival rate of shrimp in all experimental feeds was improved, but was not significant. Shrimp fed with C. vulgaris at different concentrations showed significantly (P < 0.05) higher antioxidant activity. The study showed that shrimp fed with a concentration of 30 g/kg C. vulgaris in the diet showed significantly (P < 0.05) highest disease resistance against the pathogen tested. Hence, dietary C. vulgaris might be used to improve growth performance, feed utilization, body composition, and immune biomarker responses, leading to disease resistance in cultured shrimp.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aquaculture continues to be a significant food-producing industry (Hendam et al. 2023) and source of income for millions of people worldwide. Shrimp farming has evolved rapidly and is the best source of food production in many nations (Eissa et al. 2021), leading to the most profitable project in the mariculture industry (Eissa et al. 2022). Whiteleg shrimp (Litopenaeus vannamei) is considered the most giant farmed tropical shrimp (Eissa et al. 2023a), which shares 83.1% of shrimp production global yield compared with other shrimp (FAO. The State of World Fisheries and Aquaculture 2020), probably due to its survival rate, rapid growth, and disease resistance (Eissa et al. 2022). In addition, L. vannamei has favorable biological features for selective breeding, including its lower need for dietary protein, microbial particle cropping, the flexibility to higher stocking density culture, a wider tolerance, and relative ease of domestication (Emerenciano et al. 2022).
Increasing the immunological state of shrimp via the nutritional addition of immunostimulants is one of the advantageous techniques. Managing immunostimulants by dietary addition increases growth performance, immune system responses (Alagawany et al. 2021), and resistance to infections in shrimp, minimizing the negative environmental stressors (Wang et al. 2013). Also, they offer economic and effective techniques for stability and increase in the production system (Kela 2022), in addition to the cost-efficiency of aquaculture practices by reducing expensive disease control methods. Shrimps of higher quality are more prolific and efficient, increasing growth and feeding efficiency (Gian et al. 2021). To promote the expansion and sustainability of shrimp farming, the need for healthier diets has developed a demand for protein-rich sources (Tacon et al. 2000).
Chlorella (Chlorella vulgaris) is among the most common microalgae used in aquaculture as a direct feed or inserted into foods as an additive for different organisms (Sukri et al. 2016). This microalgae has high essential nutrients, e.g., vitamins, pigments, proteins, and other growth elements (Ajiboye et al. 2012). It has a high lipid concentration, polysaccharides, minerals, and other substances, including several physiological procedures (Xu et al. 2014). Moreover, the compound beta-1,3-glucan is an operative immunostimulant that decreases blood lipids and free radical hunting (Sahoo et al. 2008). Due to these nutritional values, its application is considered a sustainable and friendly ecologically source in aquaculture industries (Ahmad et al. 2020; Safari et al. 2022). Feeding microalgae (C. vulgaris) as a feed additive has shown outstanding growth performance, digestibility, digestive enzyme activity, and antioxidant activity results in crayfish (Safari et al. 2022, 2021; Omid et al. 2022), giant prawns (Gian et al. 2021), Pacific white shrimp, and green tiger prawn (Al-Musalam et al. 2014). Furthermore, C. vulgaris as a food additive has been applied successfully in Nile tilapia, olive flounder, carp, and shrimp (Shi et al. 2017). Adding C. vulgaris to food enhances growth, survival rate, and immune responses (Radhakrishnan et al. 2015; Maliwat et al. 2017); moreover, it augments antioxidant enzymatic activity and fat digestion (Rahimnejad et al. 2016).
While being touted as having advantages, C. vulgaris is said to have negative effects when added to diets in greater amounts. Moreover, due to its rigid cell wall, it may be difficult for digestive enzymes to access the intracellular components necessary for efficient digestion and assimilation (Ahmad et al. 2020). Thus, the present study aims to investigate the effect of different doses of C. vulgaris as a feed additive on growth performance, food consumption, and the composition of the body, the immunological response, and histopathological improvement of Litopenaeus vannamei (Pacific white shrimp).
Materials and methods
Experimental design
This research was conducted at Private Shrimp Aquaculture Farm in Damietta, Egypt. After 2 weeks of acclimatization, 300 healthy L. vannamei with an initial mean weight of 4.00 ± 0.04 g were randomly selected and equally allocated into five groups of triplicate in 1 m3 hapa (20 shrimps/hapa). Experimental shrimp groups were fed C. vulgaris inclusion diets (0, 15, 20, 25, and 30 g/ kg diet) for 56 successive days as a feeding trial.
Experimental feeds and feeding protocol
In this study, the method described by Toften and Jobling (Toften and Jobling 1997) was followed to formulate five experimental diets supplemented with dried C. vulgaris powder that was purchased from the National Research Centre, Egypt. Briefly, a powder of dried C. vulgaris was combined with sterile distilled water before combining with the basal diet. The diets were made by spraying a chlorella water suspension onto the surface of the feed pellets and drying them in a cool, dry place. The amount of dried C. vulgaris powder in was 0, 15, 20, 25, and 30 g/kg feed, respectively, including: T0 = 0 g/kg as control, T1 = 15 g/kg, T2 = 20 g/kg, T3 = 25 g/kg, and T4 = 30 g/kg. All diets contained almost 39% protein and 11% lipid (Table 1). Shrimps were fed at the satiation level 3 times daily (6:00, 12:00, and 18:00).
Water quality parameters
Along the experiment, water quality parameters including temperature, pH, dissolved oxygen, and salinity were measured regularly (Table 2) using a SensoDirect-150 Multi Meter (Lovibond, Tintometer Limited, Amesbury, UK). The total ammonia nitrogen (TAN) was also measured using a HANNA HI 96715-11 Ammonia Medium Range photometer (HANNA, Nusfalau, Romania). The unionized ammonia (NH3) was measured following Boyd (Boyd 2019).
Growth performance assay
The shrimps from all experimental groups were taken fortnightly to measure the growth performance parameters. Three shrimps were collected randomly from each replicate hapa (9 shrimp/group) during sampling. The studied parameters including average daily gain (ADG), specific growth rate (SGR), weight gain (WG), survival rate (SR); and feed utilization, the average feed intake (FI), and feed conversion ratio (FCR) were measured (Eissa et al. 2023b).
-
ADG (g): {(Final Weight-Initial Weight) / number of days}
-
SGR (%): (ln final weight- ln initial weight / number of days) × 100
-
WG (g): Final Weight-Initial Weight
-
SR (%): {(Final Number of shrimp / Initial Number of shrimp) × 100}
-
FI (g): The quantity of feed given during the trial
-
FCR: (Total Feed Consumption / Weight Gain of shrimp).
Proximate body composition of experimented shrimps
At the end of the feeding trial, three shrimps were collected randomly from each hapa (9 shrimp/group) to analyze the proximate body composition (moisture, protein, lipid, and ash) using the standard protocols of the Association of Official Analytical Chemists (AOAC (Association of Official Analytical Chemists), 2000).
Immune biomarker analysis
Sample collection
Examined shrimps were caught and anesthetized with 0.2 mL/L clove oil solution before hemolymph collection. A sterile syringe (25-G 13-mm needle) was used to draw hemolymph (100−l) samples from the first abdominal segment of the ventral sinus of five shrimps per group. Before hemolymph extraction, a precooled (4 °C) anticoagulant solution was put into the syringe (0.114 M trisodium citrate, 450 mM NaCl, 10 mM KCl, 10 mM HEPES at pH 7.4) (Nonwachai et al. 2010). Total hemocyte count (THC), respiratory burst, and phagocytosis activities were estimated using an anticoagulant-hemolymph mixture. The other portion of hemolymph samples was collected and stored on ice in Eppendorf tubes for coagulation and then centrifuged instantly at 800 rpm for 10 min at 4 °C. Finally, supernatants were collected and kept at 20 °C for additional lysozyme and phenoloxidase (PO) activity testing. The hemolymph samples were centered at 3000 rpm and 4 °C for 10 min to assess antioxidant enzymes and oxidative stress markers. After removing the supernatant, the pellet was resuspended in 3 ml of 0.9% NaCl and centrifuged again. This pellet was reconstituted using 2 mL of pure water. The resulting hemocyte was stored at 4 °C.
Total hemocyte counts
The anticoagulant-hemolymph mixture (50 µL) was diluted by 150 μL of formaldehyde (4%), and 20 μL of the mixture was situated on a hemocytometer (Neubauer) to conclude THC by a microscope. THC counts were expressed as log cell/milliliter of hemolymph, calculated as follows:
THC (cells/mL) = count×104 × dilution factor (Blaxhall and Daisley 1973).
Phagocytosis assay
The phagocytosis assay was modified slightly according to a previous protocol (Itami et al. 1992). Obtained hemocytes from shrimps have been washed in prawn saline (a solution of MgCl2-6H2O 1.0 g, NaCl 28.4 g, MgSO4-7H2O 2.0 g, KCl 0.7 g, CaCl2-2H2O 2.25 g, HEPES 2.38 g/L, and glucose 1.0 g), and the quantity of cells was modified (1×106 cells/ml). Cell suspension (200 μL) was injected into the coverslip. For 20 min, the cell suspension was withdrawn and then washed three times using shrimp saline. Two hours was spent incubating 2 mL of Candida albicans fungal solution. C. albicans was then eliminated, and the suspension was rinsed five times using prawn saline (5×108 cells/mL) before methanol fixation (100%). The coverslip was stained with Giemsa before being placed using paramount slide solution mounting. Each sample was examined for two hundred hemocytes. The phagocytic index and activity were determined using the method shown below:
Respiratory burst assay
The respiratory burst activity was evaluated according to a previous protocol (Song and Y.T. 1994) based on spectrophotometric detection of formazan from the nitroblue tetrazolium, NBT-O2− redox reaction. Fifty microliters of a hemolymph anticoagulant was incubated with 50 µL of the balanced salt solution prepared by Hank without phenol red (MCHBSS, Sigma). A 96-well microtiter plate has been incubated at room temp also under humid conditions. For 30 min, the supernatants were discarded, and 50 L of MCHBSS medium was added. After, 50 µL of 0.3% NBT in a suitable medium was promptly provided for every individual. Following 2 h of incubation, supernatants were withdrawn, and 200 µL of methanol was added to the hemocytes, which were then fixed, twice washed using methanol (70%), and, finally, dried. Formazan has been accumulated in 120 L of 2 M KOH and 140 L of DMSO (dimethyl sulfoxide; Sigma, USA). After mixing the wells’ contents, a spectrophotometer was used to measure the absorbance at 620 nm.
Lysozyme activity
The lysozyme activities were measured using the turbidimetric method (Eissa et al. 2023b). Briefly, 0.2 mg of Micrococcus lysodeikticus (MI, SIGMA) powder to 1 mL of 1/15M sodium phosphate buffer (pH 6.2) was placed in 96-well plates, and 25 μL of serum sample was added to each well. The reduction in absorbance at 540 nm using a microplate spectrophotometer (Bio-Rad 680, USA) was measured between 0.5 and 4.5 min at 25 °C. One unit of lysozyme activity was defined as the amount of sample causing a reduction in absorbance at 0.001/ min.
Phenoloxidase activity
The method described by Supamattaya et al. (Supamattaya et al. 2000) with some modifications was used to determine the phenol oxidase (PO). Briefly, the hemolymph-anticoagulant combination was rinsed on three occasions with prawn saline, then followed by centrifugation at 4 °C for 10 min at 1000 rpm and 4 °C, preparation of hemocyte lysate by sonicating hemocytes into a buffer of cacodylate (0.45 M sodium chloride, 0.01 M calcium chloride, pH 7.4; 0.01 M sodium cacodylate, and 0.26 M magnesium chloride; pH 7.0) for 5 s at 30 amplitudes, then centrifuging the suspension for 20 min at 10,000 rpm (4 °C) followed by collecting supernatant. Next, 200 μL of 0.25% trypsin in cacodylate buffer was added to 200 μL of hemocyte lysate, followed by 200 μL of the substrate containing 4 mg/mL of l-dihydroxyphenylalanine. Dopachrome absorbance was used to determine the enzyme activity at 490-nm wave length. The rise in optimal density per minute/milligram of protein has been used to determine the phenol oxidase’s activity.
Challenge assay against Vibrio parahaemolyticus
The challenge assay was performed (Eissa et al. 2023b) at the end of 56 days of the feeding trial. The experimented shrimps were challenged against a pathogenic strain of Vibrio parahaemolyticus that was previously isolated from diseased L. vannamei (El-Barbary and Hal 2016). The bacterial strain was grown for 24 h at 28 °C in Brain Heart Infusion (BHI) broth with 2% NaCl. The bacterial suspension was centrifuged, and the bacterial pellet was washed several times with a sterile saline solution (1.5% NaCl). Afterward, the pellet was resuspended to a final concentration of 107 CFU/mL that was measured spectrophotometrically at an optical density of 600 nm.
A total of 30 treated shrimps from each experimental groups (T0, T1, T2, T3, and T4) were stocked in 15 transparent plastic aquariums (60 × 35 × 30 cm3) in replicates. Treatments and control were exposed to the virulent V. parahaemolyticus at a concentration of 107 CFU/mL at 28 °C via immersion (Balcázar et al. 2007) in water mixed with the prepared bacterial suspension for 24 h. During the first 24 h, water was not changed to ensure the infection.
Challenged shrimps were monitored for 15 days until the cessation of the mortalities (Rengpipat et al. 1998), and any dead shrimp were collected. On TCBS (thiosulfate-citrate-bile salts-sucrose agar), the pathogenic V. parahaemolyticus strain was reisolated from the hepatopancreas of a dead shrimp. The mortality percentage was calculated according to the following equation: Mortality rate (%) = (number of dead shrimp/total number of shrimp) × 100.
Following the challenge period, the hemolymph from the infected live shrimps was collected to validate the immunology of shrimp groups treated with different concentrations of C. vulgaris by the method described above (paragraph 2.6).
Histolopathological study
After challenged with V. parahaemolyticus, the shrimp’s hepatopancreas and muscle samples were taken from the experimental groups and fixed in 10% buffered formalin for 48 h. The histopathological steps were (i) dehydration by ascending grade of alcohol; (ii) cleared and blocked by Paraplast wax; (iii) cutting with microtome at 4 μ of thickness; and (iv) finally, stained the prepared slides with hematoxylin and eosin dyes. Then examined, the stained slides by a bright field microscope (Leica, UK) (Eissa et al. 2023b).
Statistical analysis
All the raw data were analyzed using a one-way analysis of variance (ANOVA) in SPSS (version 26). The differences between treatment means were analyzed using multiple comparisons and Duncan’s test (Steel and Torrie 1980) to determine whether they were significant at the 95% confidence interval.
Results
Growth performance and feed utilization
The inclusion of different concentrations of dried C. vulgaris powder in the shrimp feed improved significantly (P<0.05) the growth performance and feed utilization of the Pacific white shrimp compared to the control (Table 3). The higher amount of supplementation with dried C. vulgaris exhibited higher growth performance and feed utilization. However, there was no significant (P<0.05) difference between shrimps fed with 25 g/kg (T3) and 30 g/kg (T4) of dried C. vulgaris powder. Feeding treatments T1 to T4 showed significantly (P<0.05) specific growth performance (SGR) of shrimps compared to the control. The survival rates were significantly (P<0.05) highest also in T4 (82.33%) and T3 (82.19%) feeding treatments, followed by T2 (78.10%), T1 (77.67%), and T0 (68.23%).
Alike the growth performance and survival rate, supplementation with dried C. vulgaris enhanced the feed utilization indices (feed intake and feed conversion ratio). The highest feed utilization was observed in shrimp under the T4 treatment, followed by T3, T2, T1, and T0 treatments.
Proximate shrimp body composition
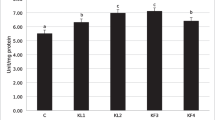
Shrimps fed with different concentrations of dried C. vulgaris additive improved significantly (P<0.05) the body content of protein and ash in shrimps compared with the control. There were no significant differences in protein content among the shrimps fed with different concentrations of dried C. vulgaris supplements. On the other hand, C. vulgaris diets (P< 0.05) considerably declined lipid content, unlike the control (Table 4). Shrimps under T4 feeding treatment showed the lowest (P<0.05), with no significantly (P<0.05) different with the T3 shrimp group.
Immune biomarker analysis
The values of the non-specific cellular immune measures (total hemocyte count, phagocytic activity, respiratory brush activity in comparison to the humoral immunological parameters, serum lysozyme, and phenol oxide activity) are presented in Table 5. Shrimp fed C. vulgaris diets showed higher significantly different (P< 0.05) values of THC, RB activity, lysozyme activity, phenol oxide activity, and phagocytic activity indices in comparison to the control non-supplemented group.
Challenge assay
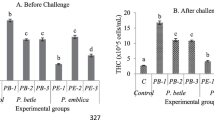
During the challenge period, the cumulative mortality rate in percentage, the hemocyte count (THC), respiratory burst (RB) activities, lysozyme activities (Lys), and phenol oxide activities (PO) were determined. The results are given in Figs. 1 and 2, respectively.
The cumulative mortality rate in the shrimp groups fed with different C. vulgaris inclusion diets and infection with the virulent V. parahaemolyticus was lower in the T4 shrimp group (10%), followed by the T3 (13%), T2 (30%) and T1 (37%) treatment groups, in a dose-dependent manner (Fig. 1) 15 days after the challenge period. All these feeding treatments were significantly (P < 0.05) lower than the control (T0, 53%). Additionally, Fig. 2 exhibits the status of dietary C. vulgaris supplement in different concentrations in shrimp feed after the end of the challenge period. It was compared with the pre-challenge period. The status of THC, RB, Lys, and PO was significantly reduced between pre- and post-challenge periods. However, the reduction rates were significantly (P < 0.05) lower compared to the control. The status of total hemocyte counts (THCs) and respiratory burst (RB) activities in post-challenge period was significantly (P < 0.05) different between the shrimp groups fed in T1 and T2 treatments (Fig. 2). However, there were no significant (P < 0.05) differences in T3 and T4 shrimp groups. T1 and T2 shrimp groups had no significant (P < 0.05) difference in lysozyme (Lys) and phenol oxide (PO) activities, a similar occurrence in shrimps’ groups T3 and T4 after 15 days of the challenge period.
Histolopathological examination
These histological responses were to ameliorate the tissues of hepatopancreases in the L. vannamei groups treated with different concentrations of dietary C. vulgaris dried powder (Fig. 3). The highest improvement occurred in the T3 shrimp group (Fig. 3d), followed by T4 (Fig. 3e), T2 (Fig. 3c), T1 (Fig. 3b), and T0 (Fig. 3a) treatment groups after resistance period against V. parahaemolyticus. Figure 4 demonstrates the refinement of shrimp muscle caused by dietary C. vulgaris supplement in shrimp feed after ending the challenge period. Better improvement of the myofibers and disappearance of myofiber tearing occurred in the T3 shrimp group (Fig. 4d), followed by T4 (Fig. 4e), T2 (Fig. 4c), T1 (Fig. 4b), and T0 (Fig. 4a) treatment groups after resistance period against V. parahaemolyticus. On the other hand, the best tissues of hepatopancreas and muscles that improved by C. vulgaris diets against the resistance of V. parahaemolyticus (Figs. 3d and 4d) that compared with the control after challenged (Figs. 3a and 4a).
Photomicrograph of histological plates of shrimp hepatopancreases which fed on the different doses of diet. a Control (T0) after challenge, b T1, c T2, d T3, and e T4. Tubules (T), tubules’ lumens (Lu), vacuoles (V), B-cell (thin arrows), F-cell (thick arrows), R-cells (asterisks), and some of hepatopancreas sloughing lining (stars) appeared. H&E, and Bar → 100 µm
Photomicrograph of histological plates of shrimp muscles which fed on the different doses of diet. a Control (T0) after challenge T0, b T1, c: T2, d T3, and e T4. Striated muscle fibers (S), muscle fibers (My), muscle nucleus (n), loose connective tissues (thin arrows), some myofiber tearing (asterisks), and thickness of muscle bundles (head arrows). H&E, and Bar → 50 µm
Discussion
This study’s results revealed that including dietary C. vulgaris in the shrimp feed enhanced the growth performance and health status of the Pacific white shrimp, L. vannamei. The performance trend was in T4 ≥ T3 > T2 ≥ T1 > T0, meaning the higher the dose of dietary C. vulgaris, the higher the performance in growth and health status of L. vannamei. In fact, dietary C. vulgaris is morphologically a single cell, but it can form colonies up to a maximum of 64 cells (Safi et al. 2014). Hence, the higher dose of dietary C. vulgaris used in this study might produce the highest number of cells that enhanced the growth performance and health status of L. vannamei. Adding dietary C. vulgaris to the shrimp diet can produce some essential nutrients that could enrich the water quality parameters during the feeding trial (Usha et al. 2021). Nevertheless, little is so far known about Chlorella meal’s potential uses as an ingredient in shrimp food. The present study demonstrated the efficacy of integrating microalgae into the diet of aquaculture shrimp. These findings will open up a new option for organic shrimp feeds for aquaculture nutritionists working with shrimp, as chlorella is a sustainable solution devoid of toxic chemicals and antibiotics (Aly et al. 2023) that aid in sustainability in this sector.
Compared to all feeding treatments tested in the present study, the nutritional supplements of 30 g/kg C. vulgaris considerably enhanced the performance of growth and feed consumption of investigated shrimp in the present research. This enhancement might be attributable to growth factors like adequate macronutrient volume and bioactive substances (Pakravan et al. 2018). C. vulgaris is known as mostly a source of protein because of its excessive composition of amino acids and vital vitamins and minerals (Becker 2007). The results of the present study were relatively similar to those previous findings of Radhakrishnan et al. (Radhakrishnan et al. 2015) and Pakravan et al. (Pakravan et al. 2018).
High survival rates were observed in L. vannamei, especially in shrimp fed with high doses of Chlorella. This may be due to increased immunity, which is consistent with what has been mentioned by Anjel et al. (Anjel et al. 2010). Also, improving the survival rate may result from some functional composites in Chlorella as amino acids, pigments, fatty acids, and minerals (Ahmad et al. 2020).
Moreover, the body analysis data showed no statistical variation in moisture (P < 0.05). However, protein and ash levels rose in shrimp given Chlorella, but lipid levels fell. The results paralleled what has been reported by Sukri et al. (Sukri et al. 2016). Elevated body protein may result from Chlorella’s increased protein production and the conversion effectiveness of eaten food into body proteins, increasing muscle building (Lara-Flores and Olvera-Novoa 2013).
In general, the stability of shrimp aquaculture depends on disease management and the immunological response of shrimp, which are essential indicators in determining the health state of shrimp (Al-Musalam et al. 2014). The immune system of shrimp consists only of cellular and hormonal responses (Roy et al. 2020).
In shrimp given Chlorella additive, the THC, RB activity, Lys activity, PO activity, phagocytic activity percent, and phagocytic index are increased significantly compared to the control. Improvement responses of immunity in shrimp food that contains Chlorella may be due to the presence of an actual number of polysaccharides in Chlorella, which are energetic immunostimulants (Huang et al. 2006), in addition to improving immune function for different species (Maliwat et al. 2017). Additionally, enhanced immunity may be attributed to the interaction between the Chlorella cell and the beneficial intestinal bacteria that can increase the activity of useful bacteria in the digestive system and their contribution to the production of organic acids.
These organic acids have positive impacts on food quality (reduction in dangerous bacteria intake) and stomach function (increase in the activity of enzymes and solubility of minerals) (Safari et al. 2021), performance intestine (elevation in digestibility of nutrient), and characters of feces (reductions in phosphorus load and microbiota) which advance of immune responses (Safari et al. 2022).
The current study found that C. vulgaris supplementation improved the immune responses of Pacific white shrimp to Vibrio parahaemolyticus infection. This study’s increasing value of immune responses was observed through total hemocyte count, pro-PO activity, and respiratory burst activity. In this study, the increase in total hemocytes after feeding supplementation occurred as a response of the shrimp’s immune system to a foreign particle. Hemocytes are critical components of crustaceans’ immune systems. One of the indicators for determining shrimp stress and health status is the fluctuating hemocyte count. Hemocytes are responsible for recognizing foreign particles that enter the shrimp’s body and responding to them through a variety of mechanisms, including phagocytosis, encapsulation, intracellular signaling cascades, and nodular aggregation (Rodriguez and Moullac 2000).
Total hemocytes decreased after the pathogen’s infection due to hemocyte migration from the circulating system of the body to tissues where many cells were infected (Yeh et al. 2009). The decrease in THC in the immune system indicated that the cellular immune arm worked on the infected areas through wound healing activities such as cell clumping, initiation of the coagulation process through the release of plasma clotting factors, and carrying and releasing factors in the phenoloxidase system (Smith et al. 2003). Furthermore, the cell apoptosis mechanism in shrimp hematopoietic tissues caused hemocytes to decline after infection. This mechanism is linked to increased serious infection and mortality in shrimp (Yeh et al. 2009). The current study is combined with a previous study that found that the hemocyte count decreased (p0.05) after a V. parahaemolyticus challenge test (Li et al. 2008). Additionally, Yeh et al. (Yeh et al. 2009) discovered a comparable case, in which the pathogen-infected shrimp hemocyte decreased following the challenge test. The development of hemocytes at the site of the infection and the occurrence of cell apoptosis as a result of viral infection are thought to be the causes.
Generally, respiratory bursts are a series of cellular processes that involve the phagocytosis of foreign particles and produce reactive oxygen intermediates (ROI) or oxygen radicals as the end product (Rodriguez and Moullac 2000). The increasing RB activity following the feeding experiment demonstrated that dietary C. vulgaris improved the immune system of Pacific white shrimp. The amount of superoxide anion (O2G) produced by hyaline cells was used in this study to gauge RB activity. Superoxide anion is the first byproduct of the RB process (O2G). Additionally, subsequent reactions will produce additional ROI, including singlet oxygen (O2), hydroxyl radicals, and hydrogen peroxide (H2O2). The hydrogen peroxide can be converted into hypochlorous acid (HOCl–) by the myeloperoxidase system (MPO–H2O2–Cl) to create an antibacterial system. The oxidative stress in the cell was brought on by the stress conditions brought on by environmental factors, different types of toxic stress, and biological stress (including pathogen infection) (Castex et al. 2010). The present study is advocated this statement.
The lysozyme (Lys) level of shrimp fed C. vulgaris supplementation was significantly higher than that in the control, with the highest dose supplements (30 g/kg of shrimp feed) being the most likely due to its immunostimulant attributes. This factor influenced Lys expression against pathogenic bacteria (Antonio et al. 2010).
In this study, the responses of several shrimp immune system parameters (THC, RB, Lys, and PO) were related to the activation of the innate immune response. The interaction of shrimp pattern recognition receptors (PRRs) and pathogen-associated molecular patterns mediated the innate immune response (PAMPs) activation. This PAMPs could be polysaccharides and glycoproteins on the surface of microbes, such as lipopolysaccharide (LPS) from Gram-negative bacteria, peptidoglycan (PGN), and lipoteichoic acid (LTA) from Gram-positive bacteria, glucans from yeast cells, and polynucleotides (Wang and Wang 2013). The dietary C. vulgaris availability in the shrimp feed is expected to produce such attributes. Thus, the cumulative mortality rate is significantly lower in all supplemented shrimp feeds compared to the control.
The current study showed that Chlorella vulgaris supplementation in shrimp meal improved the hepatopancreases and muscles of Litopenaeus vannamei to Vibrio parahaemolyticus infection compared to the control after the challenge period, consistent with matching previous conclusions (Shi et al. 2017; Zahran et al. 2019). The better results for shrimp tissue enhancement in the T3, T4, T2, and T1 groups were compared with control (T0) after being challenged.
Conclusion
Supplementation with C. vulgaris in shrimp feed showed the highest growth, survival rate, body composition, immune responses, and shrimp tissue improvement. The peak results were in the high concentration of chlorella diets. There were no significant (P < 0.05) differences between T3 (25 g/kg of shrimp feed) and T4 (30 g/kg of shrimp feed). Hence, this study suggests applying dietary C. vulgaris dried powder in 25 g/kg shrimp feed.
Data availability
The data presented in this study are available on request from the corresponding author.
References
Ahmad MT, Shariff MMd, Yusoff F, Goh YM, Banerjee S (2020) Applications of microalga Chlorella vulgaris in aquaculture. Rev Aquac 12:328–346
Ajiboye OO, Yakubu A, Fand ATE (2012) A perspective on the ingestion and nutritional effects of feed additives in farmed fish species. World J Fish Marine Sci 4:87–101
Alagawany M, Taha AE, Noreldin A, El-Tarabily KA, Abd El-Hack ME (2021) Nutritional applications of species of Spirulina and Chlorella in farmed fish: a review. Aquaculture 542:736841
Al-Musalam L, Al-Ameeri A, Saheb A, Al-Yaqout A (2014) Effect of herbal feed additive on the growth, survival and immune response of green tiger prawn (Penaeus semisulcatus). Pak J Nutr 13(7):366–371
Aly SM, ElBanna NI, Fathi M (2023) Chlorella in aquaculture: challenges, opportunities, and disease prevention for sustainable development. Aquaculture International pp, 1–28. https://doi.org/10.1007/s10499-023-01229-x
Anjel J, Lara C, Morales V, De Gracia A, Suarez O (2010) Manual of best management practices for white shrimp Penaeus vannamei farming, 1st edn. OIRSA- OSPESCA. C.A, Panama
Antonio AZ, Giovanni A, Antonio S (2010) Prebiotics and probiotics in infant nutrition. In: Bioactive Foods in Promoting Health: Probiotics and Prebiotics. Elsevier Inc, pp 441–477
Association of Official Analytical Chemists (AOAC) (2000) Official methods of analysis of AOAC International, 17th ed. Gaithersburg, USA
Balcázar JL, Rojas-Luna T, Cunningham DP (2007) Effect of the addition of four potential probiotic strains on the survival of pacific white shrimp (Litopenaeus vannamei) following immersion challenge with Vibrio parahaemolyticus. J Invertebr Pathol 96(2):147–150
Becker EW (2007) Micro Algae as a Source of Protein. Biotechnol Adv 25:207–210
Blaxhall P, Daisley K (1973) Routine haematological methods for use with fish blood. J Fish Biol 5:71–81
Boyd CE (2019) Water Quality: An Introduction, 3rd edn. Aquaculture and Aquatic Sciences, Auburn University, Auburn
Castex M, Lemaire P, Wabete N, Chim L (2010) Effect of probiotic Pediococcus acidilactici on antioxidant defences and oxidative stress of Litopenaeus stylirostris under Vibrio nigripulchritudo challenge. Fish Shellfish Immunol 28:622–631
Eissa EH, Che-Zulkifli CI, El-Badawi AA, Ali MAM, Baghdady ES, Abd Al-Kareem OM, Ahmed RA (2021) Growth-promoting and immunomodulatory impacts of commercial stimulants on Kuruma shrimp, Penaeus japonicus (Bate, 1888) Juveniles. Egypt Aquat Biol Fish 25(3):607–617
Eissa EH, Ahmed NH, El-Badawi AA, Munir MB, Abd Al-Kareem OM, Eissa MEH, Hussien EHM, Sakr SS (2022) Assessing the influence of the inclusion of Bacillus subtilis AQUA-GROW® as feed additive on the growth performance, feed utilization, immunological responses and body composition of the Pacific white shrimp, Litopenaeus vannamei. Aquac Res 53(18):6605–6615. https://doi.org/10.1111/are.16129
Eissa E-SH, Ahmed, RA, Abd Elghany NA, Elfeky A, Saadony S, Ahmed NH, Sakr SE-S, Dayrit GB, Olenada CPS, Atienza AAC et al (2023a) Potential symbiotic effects of -1,3 glucan, and fructooligosaccharides on the growth performance, immune response, redox status, and resistance of Pacific white shrimp, Litopenaeus vannamei to Fusarium solani infection. Fishes 8:105. https://doi.org/10.3390/fishes8020105
Eissa EH, Alaidaroos BA, Jastaniah SD, Munir MB, Shafi ME, Abd El-Aziz YM, Bazina WK, Ibrahim SB, Eissa MEH, Paolucci M et al (2023b) Dietary effects of nano curcumin on growth performances, body composition, blood parameters and histopathological alternation in red tilapia (Oreochromis sp.) challenged with Aspergillus flavus. Fishes 8:208. https://doi.org/10.3390/fishes8040208
El-Barbary MI, Hal AM (2016) Isolation and molecular characterization of some bacterial pathogens in El-Serw fish farm Egypt. Egypt J Aquat Biol Fish 20(4):115–127
Emerenciano MG, Rombenso AN, Vieira FDN, Martins MA, Coman GJ, Truong HH, Noble TH, Simon CJ (2022) Intensification of Penaeid shrimp culture: an applied review of advances in production systems, nutrition and breeding. Animals 12(3):1–39
FAO (2020) The State of World Fisheries and Aquaculture: Sustainability in action; the state of world fisheries and aquaculture (SOFIA). FAO: Rome, Italy
Gian CF, Maliwat SF, Velasquez SMD, Buluran M, Tayamen M, Ragaza JA (2021) Growth and immune response of pond-reared giant freshwater grawn Macrobrachium rosenbergii post larvae fed diets containing Chlorella vulgaris. Aquac Fish 6:465–470. https://doi.org/10.1016/j.aaf.2020.07.002
Hendam BM, Munir MB, Eissa ME, El-Haroun E, van Doan H, Chung TH, Eissa ESH (2023) Effects of water additive probiotic, Pediococcus acidilactici on growth performance, feed utilization, hematology, gene expression and disease resistance against Aspergillus flavus of Nile tilapia (Oreochromis niloticus). Anim Feed Sci Technol 303:115696. https://doi.org/10.1016/j.anifeedsci.2023.115696
Huang X, Zhou H, Zhang H (2006) The effect of Sargassum fusiforme polysaccharide extracts on vibriosis resistance and immune activity of the shrimp, Fennero penaeus chinensis. Fish Shellfish Immunol 20:750–757
Itami T, Takahashi Y, Tsuchihira, Igusa H (1992) Enhancement of disease resistance of Kumura prawn Penaeus japonicus and increase in phagocytic activity of prawn hemocytes by oral administration of peptidoglycan and b-1, 3-glucan, Abstract only. Third Annual Asia Fisheries Symposium Singapore
Kela E (2022) Significance of immunostimulants in aquaculture: a review. J Aquac Fish 6:046
Lara-Flores M, Olvera-Novoa MA (2013) The use of lactic acid bacteria isolated from intestinal tract of Nile tilapia (Oreochromis niloticus), as growth promoters in fish fed low protein diets. Lat Am J Aquat Res 41(3):490–497
Li J, Tan B, Mai K et al (2008) Immune responses and resistance against Vibrio parahaemolyticus induced by probiotic bacterium Arthrobacter XE-7 in Pacific white shrimp Litopenaeus Vannamei. J World Aquac Soc 39:477–489
Maliwat GC, Velasquez S, Robil JL, Chan M, Traifalgar RF, Tayamen M, Ragaza JA (2017) Growth and immune response of giant freshwater prawn Macrobrachium rosenbergii (De Man) postlarvae fed diets containing Chlorella vulgaris (Beijerinck). Aquac Res 48:1666–1676. https://doi.org/10.1111/are.13004
Nonwachai T, Purivirojkul W, Limsuwan C, Chuchird N, Velasco M, Dhar AK (2010) Growth, nonspecific immune characteristics, and survival upon challenge with Vibrio harveyi in Pacific white shrimp (Litopenaeus vannamei) raised on diets containing algal meal. Fish Shellfish Immunol 29:298–304
Omid S, Marina P, Hamidreza AM (2022) Dietary supplementation of Chlorella vulgaris improved growth performance, immunity, intestinal microbiota and stress resis. Aquaculture 554:738138
Pakravan S, Akbarzadeh A, Sajjadi MM, Hajimoradloo A, Noori F (2018) Chlorella vulgaris meal improved growth performance, digestive enzyme activities, fatty acid composition and tolerance of hypoxia and ammonia stress in juvenile Pacific white shrimp Litopenaeus vannamei. Aquac Nutr 24(1):594–604
Radhakrishnan S, Saravana BP, Seenivasan C, Muralisankar T (2015) effect of dietary replacement of fishmeal with Chlorella vulgaris on growth performance, energy utilization and digestive enzymes in Macrobrachium rosenbergii postlarvae. Int J Fish Aquac 7(5):62–70
Rahimnejad S, Park HG, Lee SM (2016) Effects of dietary inclusion of Chlorella vulgaris on growth, blood biochemical parameters, and antioxidant enzyme activity in olive flounder, Paralichthys olivaceus. J World Aquac Soc 48:103–107
Rengpipat S, Phianphak W, Piyatiratitivorakul S, Menasveta P (1998) Effects of a probiotic bacterium on black tiger shrimp Penaeus monodon survival and growth. Aquaculture 167(3–4):301–313
Rodriguez J, Le Moullac G (2000) State of the art of immunological tools and health control of penaeid shrimp. Aquaculture 191:109–119
Roy S, Bossier P, Norouzitallab P, Vanrompay D (2020) Trained immunity and perspectives for shrimp aquaculture. Rev Aquac 12:51–70
Safari O, Paolucci M, Motlagh HA (2021) Effect of dietary encapsulated organic salts (Na-acetate, Na-butyrate, Na-lactate and Na-propionate) on growth performance, haemolymph, antioxidant and digestive enzyme activities and gut microbiota of juvenile narrow clawed crayfish Astacus leptodactylus leptodactylus. Aquac. Aquac Nutr 27:91–104
Safari O, Paolucci M, Motlagh HA (2022) Dietary supplementation of Chlorella vulgaris improved growth performance, immunity, intestinal microbiota and stress resistance of juvenile narrow clawed crayfish, Pontastacus leptodactylus Eschscholtz, 1823. Aquaculture 554:1–11
Safi C, Zebib B, Merah O, Pontalier P (2014) Morphology, composition, production, processing and applications of Chlorella vulgaris: a review. Renew Sustain Energy Rev 35:265–278
Sahoo P, Das A, Mohanty S, Mohanty B, Pillai B, Mohanty J (2008) Dietary β-1,3-glucan improves the immunity and disease resistance of freshwater prawn Macrobrachium rosenbergii challenged with Aeromonas hydrophila. Aquac Res 39:1574–1578
Shi X, Luo Z, Chen F, Wei CC, Wu C, Zhu XM, Liu X (2017) Effect of fish meal replacement by Chlorella meal with dietary cellulase addition on growth performance, digestive enzymatic activities, histology and myogenic genes’ expression for crucian carp Carassius auratus. Aquac Res 48:3244–3256
Smith VJ, Brown JH, Hauton C (2003) Immunostimulation in crustaceans: does it really protect against infection? Fish Shellfish Immunol 15:71–90
Song YL, Hsieh YT (1994) Immunostimulation of tiger shrimp (Penaeus monodon) hemocytes for generation of microbicidal substances: analysis of reactive oxygen species. Dev Comp Immunol 18:201–209
Steel RGD, Torrie JH (1980) Principles and procedures of statistics a biometrical approach. Mc- Graw-Hill Book Publishing Company, New York
Sukri S, Saad C, Kamarudin M, Yasin I (2016) Effect of different levels of Chlorella meal on growth and survival of freshwater prawns Macrobrachium rosenbergii juvenile. Songklanakarin J Sci Technol 38:641–644
Supamattaya K, Pongmaneerat J, Klowklieng T (2000) Effect of β–glucan (MacroGard®) on growth performance, immune response and disease resistance in black tiger shrimp, Penaeus monodon Fabricius. Songklanakarin J Sci Technol 22:677–688
Tacon AGJ, Forster IP (2000) Global trends and challenges to aquaculture and aquafeed development in the new millennium. In: Merican Z (ed) International aquafeed-directory and buyers’ guide 2001. Turret RAI, Uxbridge, UK, pp 4–25
Toften H, Jobling M (1997) Feed intake and growth of Atlantic salmon, Salmo salar L., fed diets supplemented with oxytetracycline and squid extract. Aquac Nutr 3:145–151
Usha SZ, Rahman MR, Sarker J, Hasan SJ et al (2021) Cultivation of Chlorella vulgaris in aquaculture wastewater as alternative nutrient source and better treatment process. Bangladesh J Vet Anim Sci 9(1):43–51
Wang YC, Chang CF, Chen HY (2013) The role of glucans in protection of shrimp against disease. In: Vetvicka V, Novac M (eds) Biology and chemistry of beta-glucan. Biology and chemistry of beta-glucan 22:173–195. https://doi.org/10.2174/9781608052608113020011
Wang XW, Wang JX (2013) Pattern recognition receptors acting in innate immune system of shrimp against pathogen infections. Fish Shellfish Immunol 34:981–989
Xu W, Gao Z, Qi Z, Qiu Z, Peng JQ, Shao R (2014) Effect of dietary Chlorella on the growth performance and physiological parameters of gibel carp, Carassius auratus gibelio. Turk J Fish Aquat Sci 14:53–57
Yeh SP, Chen YN, Hsieh SL, Cheng W, Liu CH (2009) Immune response of white shrimp, Litopenaeus vannamei, after a concurrent infection with white spot syndrome virus and infectious hypodermal and hematopoietic necrosis virus. Fish Shellfish Immunol 26:582–588
Zahran E, Awadin W, Risha E, Khaled AA, Wang T (2019) Dietary supplementation of Chlorella vulgaris ameliorates chronic sodium arsenite toxicity in Nile tilapia Oreochromis niloticus as revealed by histopathological, biochemical and immune gene expression analysis. Fish Sci 85:199–215. https://doi.org/10.1007/s12562-018-1274-6
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
The experimental design, methodology, software E.H.E., R.M.A., A. E, Y. M. A., M. B.M., S.D. J., B.A. A,and A.F.,Conceptualization, visualization, method-ology, E.H.E., M. E. S., N.N. B. A., A.F., H.S.D., E.M.O., M.E. A., O.H. E, M.E. H. E., N.I. E.,, and A.F.; software, validation, formal analysis E.H.E., R.M.A., A. E, Y. M. A., and N.I. E.; investigation, data curation, M.E. A., O.H. E, M.E. H. E., N.I. E.,writing—original draft preparation, E.H.E., R.M.A., A. E, Y. M. A., M. B.M., S.D. J., B.A. A, M. E. S.; writing—review and editing, S.D. J., B.A. A, M. E. S., N.N. B. A., H.S.D.; supervision, E.H.E.; project administration, E.H.E..; funding acquisition, S.D. J. All authors have read and approved to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The research was carried out in accordance with the international standards for treating and utilizing animals in scientific experiments, and the Institutional Animal Treatment and Use Committee (IACUC) at Suez Canal University, Egypt, and the management standard operating procedures that are in conformity and comply with EU and national regulations for animal experimentation specific legislation covering the use of animals for scientific purposes (adapted Directive 2010/63/EU on 22 September 2010).
Informed consent
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Additional information
Handling Editor: Brian Austin
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eissa, ES.H., Aljarari, R.M., Elfeky, A. et al. Protective effects of Chlorella vulgaris as a feed additive on growth performance, immunity, histopathology, and disease resistance against Vibrio parahaemolyticus in the Pacific white shrimp. Aquacult Int 32, 2821–2840 (2024). https://doi.org/10.1007/s10499-023-01298-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01298-y