Abstract
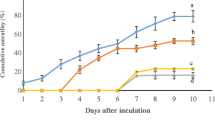
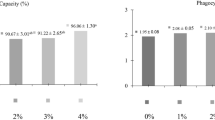
This study investigated the effects of prolonged administration of immunostimulant biomass produced by the bacteria Rubrivivax gelatinosus on the innate immune system of pacu Piaractus mesopotamicus infected with Aeromonas hydrophila. Fish were supplemented for 2 months with diets containing 0 g kg−1 biomass (TR1), 0.5 g kg−1 biomass (TR2) and 1.5 g kg−1 biomass (TR3). Haematological, biochemical and immunological analyses were carried out before biomass supplementation and 24 h after the triggering of its immune system with sub-lethal doses of A. hydrophila. As planned, no mortality was recorded in any group. After the bacterial challenge, fish from all groups showed a decrease (p < 0.05) in thrombocytes and lymphocytes and increase (p < 0.05) in monocytes, neutrophils and positive granular leucocyte (PAS-GL). Also, infected fish showed a decrease (p < 0.05) in glucose, protein, albumin and globulin and an increase (p < 0.05) in ALT and AST levels. Burst was higher (p < 0.05) after infection. The most relevant results were the increase (p < 0.05) of about 100% in monocytes and 50% in neutrophils in the group with the highest biomass supplementation compared with the control. These results suggest that incorporation of R. gelatinosus biomass into the diet for 60 days before exposure to a stress factor increases important cells involved in defence in the innate immune system.
Similar content being viewed by others
References
Akhter N, Wu B, Memon AM, Mohsin M (2015) Probiotics and prebiotics associated with aquaculture: a review. Fish Shellfish Immunol 45(2):733–741
AOAC (1990) Official method of analysis. Association of Official Analytical Chemists, Washington
Auffray C, Sieweke MH, Geissmann F (2009) Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol 27:669–692
Aydin S, Gültepe N, Yildiz H (2000) Natural and experimental infections of Campylobacter cryaerophila in rainbow trout: gross pathology, bacteriology, clinical pathology and chemotherapy. Fish Pathol 35:117–123
Biller-Takahashi JD, Takahashi LS, Saita MV, Gimbo RY, Urbinati EC (2013) Leukocytes respiratory burst activity as indicator of innate immunity of pacu Piaractus mesopotamicus. Braz J Biol 73(2):425–429
Das A, Sahoo PK, Mohanty BR, Jena JK (2011) Pathophysiology of experimental Aeromonas hydrophila infection in Puntius sarana: early changes in blood and aspects of the innate immune-related gene expression in survivors. Vet Immunol Immunopathol 142(3):207–218
Demers NE, Bayne CJ (1997) The immediate effects of stress on hormones and plasma lysozyme in rainbow trout. Dev Comp Immunol 21(4):363–373.
Dügenci SK, Arda N, Candan A (2003) Some medicinal plants as immunostimulant for fish. J Ethnopharmacol 88(1):99–106
Gallani SU, Valladão GMR, Ponsano EHG, Pilarski F (2017) Rubrivivax gelatinosus biomass as an immunostimulant for pacu Piaractus mesopotamicus. Aquac Res 48(9):4836–4843
Gamboa-Delgado J, Márquez-Reyes JM (2016) Potential of microbial-derived nutrients for aquaculture development. Rev Aquac 10(1):224–246
Giri SS, Chi C, Jun JW, Park SC (2016) Use of bacterial subcellular components as immunostimulants in fish aquaculture. Rev Aquac 10:474–492. https://doi.org/10.1111/raq.12182
Goldenfarb PB, Bowyer FP, Hall E, Brosius E (1971) Reproducibility in the hematology laboratory: the microhematocrit determination. Am J Clin Pathol 56:35–39
Grassi TLM, Espírito Santo EF, Siqueira Marcos MT, Cavazzana JF, Oliveira DL, Bossolani ILC, Ponsano EHG (2015) Bacterial pigment for Nile tilapia feeding. Aquac Int 24:647–660
Havixbeck JJ, Rieger AM, Wong ME, Hodgkinson JW, Barreda DR (2016) Neutrophil contributions to the induction and regulation of the acute inflammatory response in teleost fish. J Leukoc Biol 99(2):241–252
Hesser EF (1960) Methods for routine fish hematology. Prog Fish-Cult 22(4):164–171
Liaaen-Jensen S, Jensen A (1971) Quantitative determination of carotenoids in photosynthetic tissues: total carotenoid content. In: Pietro AS (ed) Methods in enzimology: photosynthesis and nitrogen part A. Elsevier, New York
Lima LKF, Ponsano EHG, Pinto MF (2011) Cultivation of Rubrivivax gelatinosus in fish industry effluent for depollution and biomass production. World J Microbiol Biotechnol 27(11):2553–2558
Luu NT, Madden J, Calder PC, Grimble RF, Shearman CP, Chan T, Dastur N, Howel WM, Rainger GE, Nash GB (2007) Dietary supplementation with fish oil modifies the ability of human monocytes to induce an inflammatory response. J Nutr 137(12):2769–2774
Magnadóttir B (2006) Innate immunity of fish (overview). Fish Shellfish Immunol 20(2):137–151
Mukkata K, Kantachote D, Wittayaweerasak B, Techkarnjanaruk S, Boonapatcharoen N (2016) Diversity of purple nonsulfur bacteria in shrimp ponds with varying mercury levels. Saudi J Biol Sci 23(4):478–487
Nagashima S, Kamimura A, Shimizu T, Nakamura-Isaki S, Aono E, Sakamoto K, Ichikawa N, Nakazawa H, Sekine M, Yamazaki S, Fujita N, Shimada K, Hanada S, Nagashima KV (2012) Complete genome sequence of phototrophic betaproteobacterium Rubrivivax gelatinosus IL144. J Bacteriol 194(13):3541–3542
Neumann NF, Staffor JL, Barredam D, Ainsworth AJ, Belosevic M (2001) Antimicrobial mechanisms of fish phagocytes and their role in host defense. Dev Comp Immunol 25(8):807–825
Nevejan N, Schryver P, Wille M, Dierckens K, Baruah K, Van Stappen G (2016) Bacteria as food in aquaculture: do they make a difference? Rev Aquac 10(1):180–212
Polonio LB, Ponsano EHG, Pinto MF, Garcia-Neto M (2010) Utilisation of bacterial (Rubrivivax gelatinosus) biomass for egg yolk pigmentation. Anim Prod Sci 50(1):1–5
Ponsano EHG, Lacava PM, Pinto MF (2002) Isolation of Rhodocyclus gelatinosus from poultry slaughterhouse wastewater. Braz Arch Biol Technol 45(4):445–449
Ponsano EHG, Lacava PM, Pinto MF (2003) Chemical composition of Rhodocyclus gelatinosus biomass produced in poultry slaughterhouse wastewater. Braz Arch Biol Technol 46(2):143–147
Ponsano EHG, Pinto MF, Garcia-Neto M, Lacava PM (2004) Performance and color of broilers fed diets containing Rhodocyclus gelatinosus biomass. Braz J Poultry Sci 6(4):237–242
Ponsano EH, Paulino CZ, Pinto MF (2008) Phototrophic growth of Rubrivivax gelatinosus in poultry slaughterhouse wastewater. Bioresour Technol 99(9):3836–3842
Ponsano EHG, Costa AWMD, Grassi TLM, Cintra LFC, Moraes OCD, Garcia Neto M, Pinto MF (2013) Quality of egg yolks after oxycarotenoids supplementation in hens’ diets. Poult Sci 49:242–242
Ranzani-Paiva MJT, Pádua SB, Tavares-Dias M, Egami MI (2013) Métodos para análise hematológica em peixes. EdUEM, Maringá
Řehulka J (2002) Aeromonas causes severe skin lesions in rainbow trout (Oncorhynchus mykiss): clinical pathology, haematology, and biochemistry. Acta Vet Brno 71(3):351–360
Sebastião F, Furlan L, Hashimoto D, Pilarski F (2015) Identification of bacterial fish pathogens in Brazil by direct colony PCR and 16S rRNA gene sequencing. Adv Microbiol 5(6):409–424
Secombes CJ, Fletcher TC (1992) The role of phagocytes in the protective mechanisms of fish. Annu Rev Fish Dis 2:53–71
Tamamdusturi R, Yuhana M (2016) Administration of microencapsulated probiotic Bacillus sp. NP5 and prebiotic Mannan oligosaccharide for prevention of Aeromonas hydrophila infection on Pangasianodon hypophthalmus. J Fish Aquat Sci 11(1):67–76
Valladão GMR, Gallani SU, Pilarski F (2018) South American fish for continental aquaculture. Rev Aquac 10(2):351–369
Wu P, Li JZ, Wang YL, Tong QY, Liu XS, Du C, Li N (2015) Improving the growth of Rubrivivax gelatinosus cultivated in sewage environment. Bioprocess Biosyst Eng 38(1):79–84
Yang W, Li N, Li M, Zhang D, An G (2016) Complete genome sequence of fish pathogen Aeromonas hydrophila JBN2301. Genome Announc 4(1):e01615–e01615
Yu JH, Han JJ, Park SW (2010) Haematological and biochemical alterations in Korean catfish, Silurus asotus, experimentally infected with Edwardsiella tarda. Aquac Res 41(2):295–302
Zanuzzo FS, Sabioni RE, Montoya LNF, Favero G, Urbinati EC (2017) Aloe vera enhances the innate immune response of pacu (Piaractus mesopotamicus) after transport stress and combined heat killed Aeromonas hydrophila infection. Fish Shellfish Immun 65:198–205
Funding
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico CNPq (grant number 130418/2013-7) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (grant number 2013/08353-0).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gallani, S.U., Valladão, G.M.R., Kotzent, S. et al. Dietary intake of Rubrivivax gelatinosus biomass enhances phagocytic cells in tropical fish Piaractus mesopotamicus infected with Aeromonas hydrophila. Aquacult Int 27, 711–720 (2019). https://doi.org/10.1007/s10499-019-00359-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-019-00359-5