Abstract
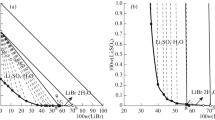
The solid-liquid phase equilibria of aqueous system containing the sulfates of lithium and potassium (Li2SO4 + K2SO4 + H2O) at T = 303.2 and 318.2 K were done by isothermal dissolution method. The phase equilibria data (solubility, density, and refractive index) of the system were determined experimentally. The corresponding solid-liquid phase diagram, density/refractive index versus composition diagrams, were plotted. There are two ternary invariant points and three crystallization regions corresponding to Li2SO4·H2O, LiKSO4, and K2SO4 in the phase diagram of system Li2SO4 + K2SO4 + H2O at 303.2 and 318.2 K. A comparision of system Li2SO4 + K2SO4 + H2O at different temperature (T = 288.2, 303.2, 318.2 and 348.2 K) shown that the double salt LiKSO4 was formed in the above mentioned temperatures, and the crystallization region of the LiKSO4 increases gradually with the increase of temperature.










Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
References
China Nonferrous Metals Industry Association (2020) Methods for chemical analysis of aluminium and aluminium alloys-part 9: determination of Lithium content-flame atomic absorption spectrometry. (GB/T 20975.9–2020. China Standards Press, Beijing, China. (in Chinese)
Cui RZ, Yang L, Wang W et al (2017) Measurements and calculations of solid-liquid equilibria in quaternary system Li2SO4 - Na2SO4 - K2SO4 - H2O at 288 K. Chem Res Chin Univ 33:460–465
Gao F, Zheng MP, Song PS et al (2012) The 273.15-K-isothermal evaporation experiment of lithium brine from the Zhabei Salt Lake, Tibet, and its geochemical significance. Aquat Geochem 18:343–356
Geological Survey US (2023) Mineral Commodity summaries. U. S. Geological Survey, Virginia
Guo YF, Liu YH, Wang Q et al (2013) Phase equilibria and phase diagrams for the aqueous ternary system (Na2SO4 + Li2SO4 + H2O) at (288 and 308) K. J Chem Eng Data 58:2763–2767
Haynes WM, Lide DR, Bruno TJ (2016) CRC handbook of chemistry and physics, 97th edn. CRC Press, Boca Raton
Institute of Qinghai Salt-Lake of Chinese Academy of Sciences (1988) Analytical methods of brines and salts, 2nd edn. Chinese Science Press, Beijing ((in Chinese))
Ji ZY, Peng JL, Yuan JS et al (2015) Stable phase equilibria in the ternary system (Na2SO4 + Li2SO4 + H2O) at 308.15 K and 313.15 K. Fluid Phase Equilib 397:81–86
Khu KY (1959) Politerma rastvorimosti v sisteme Li2SO4 - Na2SO4 - H2O. Russ. J Inorg Chem 4:1909–1911
Lerman A (2009) Saline lakes’ response to global change. Aquat Geochem 15(1–2):1–5
Lin XF, Zeng Y, Zheng ZY (2007) Study on metastable phase equilibrium of Na+, K+ // SO42– - H2O ternary system at 273 K. J Salt Lake Res 15:24–27 ((in Chinese with English abstract))
Liu HS, Jiang LS, Wu D et al (2016) Experiment and simulation study of a trapezoidal salt gradient solar pond. Acta Energiae Solaris Sinica 37(5):1227–1234 ((in Chinese with English abstract))
Lv P, Zhong Y, Meng RY et al (2015) Weighing titration analysis of the sulfate content. J Salt Lake Res 23:5–13 ((in Chinese with English abstract))
Nie Z, Wu Q, Ding T et al (2022) Research progress on the industrialization technology of lithium extraction from salt lake brine in China. Inorg Chem Ind 54:1–12 ((in Chinese with English abstract))
Ran GF, Ma HZ, Meng RY et al (2009) Rapid determination of potassium content by sodium tetraphenylboron-quaternary ammonium salt volumetric method. J Salt Lake Res 17:39–42 ((in Chinese with English abstract))
Shen W, Ren YS, Ma HJ et al (2016) Investigation of solid–liquid Equilibria on the System Na+, K+ // Cl–, SO42– - H2O and Na+, K+ // SO42– - H2O at 313.15 K. J Chem Eng Data 61:2027–2039
Wang SQ, Guo YF, Li DC (2015) Experimental determination and modeling of the solubility phase diagram of the ternary system (Li2SO4 + K2SO4 + H2O) at 288.15 K. Thermochim Acta 601:75–81
Yanko AP, Beloborodova VD (1963) Kratkie Soobshch. O Nauchn-Issled, Rabotakh
Yu XD, Yao ZH, Zhao ZX et al (2023) Phase equilibria of aqueous ternary systems Li2SO4+ Na2SO4 + H2O and Na2SO4 + K2SO4 + H2O at 303.2 K. J Chem Eng Data 68:474–482
Zeng Y, Lin XF, Yu XD (2012) Study on the solubility of the aqueous quaternary system Li2SO4 + Na2SO4 + K2SO4 + H2O at 273.15 K. Chem Eng Data 57:3672–3676
Zhang YM, Zhang ZH, Cui RZ et al (2022) Phase equilibria of LiCl - Li2SO4– H2O and Li2SO4–Na2SO4–H2O ternary systems at 333.15 K. J Salt Lake Res 30:34–41
Zhao ZX, Yao ZH, Huang Q et al (2022) Phase equilibria of aqueous quaternary system Li2SO4 + Na2SO4 + K2SO4 + H2O at 298.2 K. J Salt Lake Res 30:26–34 ((in Chinese with English abstract))
Zheng MP (2014) Saline lakes and salt basin deposits in China—Selected works of Zheng mianping. Science Press, Beijing
Zheng MP, Liu XF (2009) Hydrochemistry of salt lakes of the Qinghai-Tibet Plateau, China. Aquat Geochem 15:293–320
Zheng MP, Deng TL, Aharon O et al (2018) Introduction to Salt Lake sciences. Science Press, Beijing
Zheng MP, Xing EY, Zhang XF et al (2023) Classification and mineralization of global lithium deposits and lithium extraction technologies for exogenetic lithium deposits. China Geol 6:1–21
Solid-liquid Equilibria (SLE) of the System Containing the Sulfates of Lithium and Potassium at 303.2 and 318.2 K
Acknowledgements
This project was supported by Sichuan Science and Technology Program (No. 2022YFQ0075), Major Science and Technology Projects of Tibet Autonomous Region(XZ202201ZD0004G).
Author information
Authors and Affiliations
Contributions
Z.H.Y. Writing-Review & Editing. X.D.Y. Conceptualization, Methodology, WritingReview & Editing. Z.X.Z. Validation, Investigation, Data Curation, Writing-Original Draft. X.F. Formal analysis, Writing-Review & Editing. Y.S.Y. Formal analysis, Data Curation, Writing-Original Draft. Q.L. Data Curation, Writing-Original Draft. Y.Z. Methodology, Conceptualization, Supervision. H.J. Resources, Funding acquisition. Y.R.L. Data Curation, Resources.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yao, Z., Yu, X., Zhao, Z. et al. Solid-liquid Equilibria (SLE) of the System Containing the Sulfates of Lithium and Potassium at 303.2 and 318.2 K. Aquat Geochem (2024). https://doi.org/10.1007/s10498-023-09420-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10498-023-09420-5