Abstract
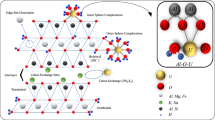
Equilibrium-kinetic modeling allows investigating metal behavior in the water–rock-organic matter system with time to evaluate anthropogenic effects on the environment. In the article, the interactions of stagnant mine drainage water of the flooded mine “Arsenic” with ore and gangue minerals were simulated using different organic matter incorporation approaches. If the model is closed to humic substances (no additional organic matter input), most fulvic acids are bound in the Fe fulvate complex. While under the removal of Fe fulvate from the model, the Cu fulvate becomes prevalent, the contribution of the fulvate complexes with Zn, Mg, and Ca also increases. This scenario simulates the organo-mineral complexes behavior well and allows identifying the sequence of metal binding to organic ligands as follows Fe > Cu > Zn > Mg > Ca. The second scenario imitates the constant input of organic matter to the model (open system regarding humic substances). The dissolved metal concentrations in the model solution are extremely high in comparison to the mine drainage water. This scenario demonstrates that excessive input of organic matter leads to the accumulation of the metals in a dissolved form and blocks the secondary mineral formation despite the faster dissolution of the primary minerals under a more acidic pH than in the first scenario. However, despite the differences between the model solution and the mine drainage water, this scenario is useful to address specific issues associated with changes in natural and anthropogenic conditions. Both scenarios show the importance of organic matter incorporation to the equilibrium-kinetic models.






Similar content being viewed by others
Data availability
The data that support the findings are published (see references) and/or available upon request.
Code availability
The license for the database and program complex for thermodynamic and kinetic modeling of geochemical processes GEOCHEQ is distributed with the terms of the developers (Mironenko et al.) upon request.
Notes
Here and after percent (%) means mole percent (mol%).
References
Besold J, Biswas A, Suess E, Scheinost AC, Rossberg A, Mikutta C, Kretzschmar R, Gustafsson JP, Planer-Friedrich B (2018) Monothioarsenate transformationkinetics determining arsenic sequestration by sulfhydryl groups of peat. Environ Sci Tech 52(13):7317–7326. https://doi.org/10.1021/acs.est.8b01542
Borisov IV (2007) Geographic basics of preservation and sustainable use of unique technogeneous-natural complexes of the Northern Ladoga area: Dissertation. Saint-Petersburg State University, Saint-Petersburg (in Russian)
Catrouillet C, Davranche M, Dia A, Coz MB-L, Demangeat E, Gruau G (2016) Does As(III) interact with Fe(II), Fe(III) and organic matter through ternary complexes? J Colloid Interface Sci 470:153–161. https://doi.org/10.1016/j.jcis.2016.02.047
Cui J, Jing C (2019) A review of arsenic interfacial geochemistry in groundwater and the role of organic matter. Ecotoxicol Environ Saf 183:109550. https://doi.org/10.1016/j.ecoenv.2019.109550
Declercq J, Oelkers EH (2014) CarbFix Report no. 281348 PHREEQC mineral dissolution kinetics database. Geoscience Environement, Toulouse.
Duffy CJ, Greenwood HJ (1979) Phase equilibria in the system MgO-MgF2-SiO2-H2O. Am Miner 64:1156–1174
Ernst R, Allen HE, Mancy KH (1975) Characteristic of trace metal species and measurements of trace metal stability constants by electrochemical techniques. Water Res 6:969–979
Fakour H, Lin TF (2014) Experimental determination and modeling of arsenic complexation with humic and fulvic acids. J Hazard Mater 279:569–578. https://doi.org/10.1016/j.jhazmat.2014.07.039
Gao J, Yang H, Li B (2016) Investigating the roles of dissolved organic matter on arsenic mobilization and speciation in environmental water. Clean Soil Air Water 44(7):818–828.
Grendal G (1896) Pitkyaranta. Brief description of Pitkyarantskoye deposit, mines and factories. Tipo-Litographiya N.A. Vineke, Saint-Petersburg (in Russian)
Gwak J-H, Jung M-Y, Hong H, Kim J-G, Quan Z-X, Reinfelder JR, Spasov E, Neufeld JD, Wanger M, Rhee S-K (2020) Archaeal nitrification is constrained by copper complexation with organic matter in municipal wastewater treatment plants. ISME J 14:335–346. https://doi.org/10.1038/s41396-019-0538-1
Higuchi M (2009) Electochromic organic-metallic hybrid polymers: fundamentals and device applications. Polym J 41:511–520. https://doi.org/10.1295/polymj.PJ2009053
Huang J-H, Voegelin A, Pombo SA, Lazzaro A, Zeyer J, Kretzschmar R (2011) Influence of Arsenate adsobtion to ferrihydrite, goethite, and boehmite on kinetics of arsenate reduction by Shewanella putrefaciens strain CN-32. Environ Sci Tech 45(18):7701–7709. https://doi.org/10.1021/es201503g
Kinniburgh DG, Milne CJ, Benedetti MF, Pinheiro JP, Filius J, Koopal LK, Van Riemsdijk WH (1996) Metal ion binding by humic acid: application of the NICA-Donnan model. Environ Sci Technol 30:1687–1698
Kolpakova M, Gaskova O (2017) Chemical modeling of maijor and trace elements complexes in Weatern Mongolian saline lakes. SGEM2017 Conf Proc 17(31):105–112.
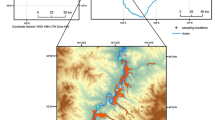
Konyshev AA, Sidkina ES, Cherkasova EV, Mironenko MV, Gridasov AG, Zhilkina AV, Bugaev IA (2020) Migration forms of heavy metals and chemical composition of surface waters in the area of arsenic mine (Pitkäranta ore district, South Karelia). Geochem Int 58(9):1068–1074. https://doi.org/10.1134/S0016702920090050
Krasintseva VV, Grichuk DV, Romanova GI, Kadukin AI (1982) Migration processes and the form of occurrence of chemical elements in the pore waters of bottom sediments in the Ivankovo reservoir. Geochem 9:1342–1354 (in Russian)
Kraynov SR, Shvarov YuV, Grichuk DV, Dobrovolskiy EV, Solomin GA, Borisov MV, Ryzhenko BN, Matveeva LI, Lyalko VI, Shvets VM (1988) Methods of geochemical modeling and forcasting in hydrogeology. Nedra, Moscow (in Russian)
Lazareva EV, Myagkaya IN, Kirichenko IS, Gustaytis MA, Zhmodik SM (2019) Interaction of natural organic matter with acid mine drainage: In-situ accumulation of elements. Sci Tot Environ 660:468–483. https://doi.org/10.1016/j.scitotenv.2018
Lemaire E, Blanc G, Schäfer J, Coynel A, Etcheber H (2006) Dissolved trace metal-organic complexes in the lot-garonne river system determined using the C18 Sep-Pak System. Aquatic Geochem 12:21–38. https://doi.org/10.1007/s10498-005-0908-3
Lenoble V, Dang DH, Loustau Cazalet M, Mounier S, Pfeifer HR, Garnier C (2015) Evaluation and modelling of dissolved organic matter reactivity toward AsIII and AsV–Implication in environmental arsenic speciation. Talanta 134:530–537. https://doi.org/10.1016/j.talanta.2014.11.053
Lepokurova OE, Ivanova IS (2014) Geochemistry of iron in organogenic water of Western Siberia, Russia. Proc Erath Plan Sci 10:297–302. https://doi.org/10.1016/j.proeps.2014.08.068
Levshina SI (2015) The role of humic acids in migration of metals in river water in Amur region. Water Res 42:810–820. https://doi.org/10.1134/S0097807815060068
Lipatnikova OA, Grichuk DV (2011) Thermodynamic modeling of the forms of heavy metals in bottom sediments on the example of the Ivankovo reservoir. Mosc Univ Geol Bull 2:51–59 (in Russian)
Mamindy-Pajany Y, Hurel C, Marmier N, Roméo M (2009) Arsenic adsorption onto hematite and goethite. C R Chimie 12:876–881. https://doi.org/10.1016/j.crci.2008.10.012
Mantoura RFC, Dickson A, Riley SP (1978) The complexation of metals with humic materials in natural waters. Estuarine Coastal Marine Sci 6:387–408. https://doi.org/10.1016/0302-3524(78)90130-5
Mironenko MV, Zolotov MY (2012) Equilibrium-kinetic model of water-rock interaction. Geochem Int 50(1):1–7. https://doi.org/10.1134/S0016702912010089
Mironenko MV, Melihova TYu, Zolotov MYu, Akinfiev NN (2008) GEOCHEQ_M: Program complex for thermodynamic and kinetic modeling of geochemical processes in rock–water–gas systems. Version 2008. Vestnik Otdelenia Nauk o Zemle RAN 1(26). (in Russian)
Moiseenko TI, Dinu MI, Gashkina NA, Kremleva TA (2012) Metal speciation in natural waters and metal complexing with humic matter. Doklady Earth Sci 442(2):267–271. https://doi.org/10.1134/S1028334X12020171
Moiseenko TI, Dinu MI, Gashkina NA, Kremleva TA (2013) Occurrence forms of metals in natural waters depending on their chemical composition. Water Res 40(4):407–416. https://doi.org/10.1134/S009780781304009X
Naymushina O, Kolpakova M, Gaskova O (2017) Chemical speciation of heavy metals in peat bog, Kokchetav mountain group. North Kazakhstan. SGEM2017 Conf Proc 17(31):113–120.
Nordstrom DK (2011) Hydrogeochemical processes governing the origin, transport and fate of major and trace elements from mine wastes and mineralized rock to surface waters. Appl Geochem 26:1777–1791. https://doi.org/10.1016/j.apgeochem.2011.06.002
Order N 552 (2016) Order of the Ministry of Agriculture of the Russian Federation of December 13, 2016 N 552 “On approval of water quality standards for fishery water bodies, including the standards of maximum permissible concentrations of harmful substances in the waters of water bodies of fishery value” (as amended on October 12, 2018) (in Russian)
Perdue EM, Beck KC, Reuter JH (1976) Organic complexes of iron and aluminium in natural waters. Nature 260:418–420
Ren J, Fan W, Wang X, Ma Q, Li X, Xu Z, Wei C (2017) Influences of size-fractionated humic acids on arsenite and arsenate complexation and toxicity to Daphnia magna. Water Res 108:68–77. https://doi.org/10.1016/j.watres.2016.10.052
Rose AL, Waite TD (2003) Kinetics of iron complexation by dissolved natural organic matter in coastal waters. Marine Chem 84:85–103. https://doi.org/10.1016/S0304-4203(03)00113-0
Rosgydromet (2017) Review of the state and pollution of the environment in the Russian federation, 2016th edn. T.M, Chernogaeva (in Russian)
Sander SG, Koschinsky A (2011) Metal flux from hydrothermal vents increased by organic complexation. Nature Geosci 4:145–150. https://doi.org/10.1038/ngeo1088
Sundman A, Karlsson T, Sjöberg S, Persson P (2014) Complexation and precipitation reactions in the ternary As(V)–Fe(III)–OM (organic matter) system. Geochim Cosmochim Acta 145:297–314. https://doi.org/10.1016/j.gca.2014.09.036
Tipping E (1998) Humic ion-binding Model VI: an improved description of the interactions of protons and metal ions with humic substances. Aquatic Geochem 4:3–47. https://doi.org/10.1023/A:1009627214459
Trüstedt O (1907) Die Erzlagerstätten von Pitkäranta am Ladoga-See. Bulletin de la Commission Géologique de Finlande 19:243–244 (in German)
Valkama M, Sundblad K, Cook NJ, Ivashchenko VI (2016) Geochemistry and petrology of the indium-bearing polymetallic skarn ores at Pitkäranta, Ladoga Karelia. Russia Miner Depos 51(6):823–839. https://doi.org/10.1007/s00126-016-0641-4
Varshal GM, Inshkirveli IS, Sirotkina IS, Kolosov IV, Koscheeva IYa (1975) About the association of fulvic acids in aqueous solutions. Geochem 10:1581–1585 (in Russian)
Varshal GM, Koscheeva IYa, Sirotkina IS, Velyuhanova TK, Inshkirveli IS, Zamokina NS (1979) Study of organic substances of surface waters and their interaction with metal ions. Geochem 4:598–607 (in Russian)
Varshal GM, Velyuhanova TK, Koscheeva IYa, Dorofeeva VA, Bauchidze NS, Kasimova OG, Maharadze GA (1983) Study of the chemical forms of elements in surface waters. J Anal Chem 38:1590–1600 (in Russian)
Wang S, Mulligan CN (2009) Effect of natural organic matter on arsenic mobilization from mine tailings. J Hazard Mater 168:721–726. https://doi.org/10.1016/j.jhazmat.2009.02.088
Wichard T, Mishra B, Myneni SCB, Bellenger JP, Kraepiel AML (2009) Storage and bioavailability of molybdenum in soils increased by organic matter complexation. Nature Geosci 2:625–629. https://doi.org/10.1038/ngeo589
Williamson MA, Rimstidt JD (1994) The kinetics and electrochemical rate-determining step of aqueous pyrite oxidation. Geochim Cosmochim Acta 58(24):5443–5454. https://doi.org/10.1016/0016-7037(94)90241-0
Yamashita Y, Nishioka J, Obata H, Ogawa H (2020) Shelf humic substances as carriers for basin-scale iron transport in the North Pacific. Scientific Reports 10:4505. https://doi.org/10.1038/s41598-020-61375-7
Zheng X, Duan C, Xia B, Jiang Y, Wen J (2019) Hydrogeochemical modeling of the shallow thermal water evolution in Yangbajing geothermal field. Tibet J Earth Sci 30(4):870–878. https://doi.org/10.1007/s12583-016-0918-7
Acknowledgements
The authors thank professor A.Yu. Bychkov (Lomonosov Moscow State University) for valuable recommendations, A.G. Gridasov for his help with the field investigations, A.V. Zhilkina (GEOKHI RAS) for the analysis of water samples.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cherkasova, E.V., Konyshev, A.A., Soldatova, E.A. et al. Metal Speciation in Water of the Flooded Mine “Arsenic” (Karelia, Russia): Equilibrium-Kinetic Modeling with a Focus on the Influence of Humic Substances. Aquat Geochem 27, 141–158 (2021). https://doi.org/10.1007/s10498-021-09393-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10498-021-09393-3