Abstract
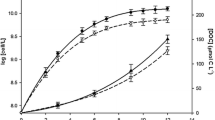
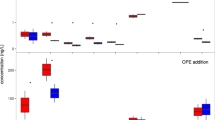
Fe(II)-Fe(III) redox behavior has been studied in the presence of catechol under different pH, ionic media, and organic compound concentrations. Catechol undergoes oxidation in oxic conditions producing semiquinone and quinone and reduces Fe(III) in natural solutions including seawater (SW). It is a pH-dependent process. Under darkness, the amount of Fe(II) generated is smaller and is related to less oxidation of catechol. The Fe(II) regeneration is higher at lower pH values both in SW with log k = 1.86 (M−1 s−1) at pH 7.3 and 0.26 (M−1 s−1) at pH 8.0, and in NaCl solutions with log k of 1.54 (M−1 s−1) at pH 7.3 and 0.57 (M−1 s−1) at pH 8.0. At higher pH values, rate constants are higher in NaCl solutions than in SW. This is due to the complexation of Mg(II) present in the media with the semiquinone that inhibits the formation of a second Fe(II) through the reaction of this intermediate with other center Fe(Cat)+.











Similar content being viewed by others
References
Anderson MA, Morel FMM (1982) The influence of aqueous iron chemistry on the uptake of iron by the coastal diatom Thalassiosira-weissflogii. Limnol Oceanogr 27:789–813
Avdeef A, Sofen SR, Bregante TL, Raymond KN (1978) Coordination chemistry of microbial iron transport compounds. 9. Stability constants for catechol models of enterobactin. J Am Chem Soc 100:5362–5370
Borer PM, Sulzberger B, Reichard P, Kraemer SM (2005) Effect of siderophores on the light-induced dissolution of colloidal iron(III) (hydr)oxides. Mar Chem 93:179–193
Borer PM, Sulzberger B, Hug SJ, Kraemer SM, Kretzschmar R (2009) Photoreductive dissolution of iron(III) (hydr)oxides in absence of organic ligands: experimental studies and kinetic modelling. Environ Sci Technol 43:1864–1870
Boye M, Nishioska J, Croot PL, Laan P, Timmermans KR, de Baar HJW (2005) Major deviation of iron complexation during 22 days of a mesoscale iron enrichment in the open Southern Ocean. Mar Chem 96:257–271
Brooksby PA, Schiel DR, Abell AD (2008) Electrochemistry of catechol terminated monolayers with Cu(II), Ni(II) and Fe(III) cations: a model for the marine adhesive interface. Langmuir 24:9074–9081
Bruland KW, Rue EL, Smith GH (2001) Iron an macronutrients in California coastal upwelling regimes: implications for diatom blooms. Limnol Oceanogr 46:1661–1674
Craig P, Shaw TJ, Miller P et al (2009) Use of multiparametric techniques to quantify the effects of naturally occurring ligands on the kinetics of Fe(II) oxidation. Environ Sci Technol 43:337–342
de Baar HJW, Boyd PW, Koale KH et al (2005) Synthesis of iron fertilization experiments: from the iron age to the age of enlightenment. J Geophys Res 110:C09S16
Dhungana S, Anthony CRIII, Hersman LE (2007) Ferrihydrite dissolution by pyridine-2, 6-bis(monothiocarboxylic acid) and hydrolysis products. Geochim Cosmochim Acta 71:5651–5660
González-Dávila M, Santana-Casiano JM, Millero FJ (2005) Oxidation of iron(II) nanomolar with H2O2 in seawater. Geochim Cosmochim Acta 69:83–93
Haber F, Weiss J (1934) The catalytic decomposition of hydrogen peroxide by iron salts. Proc R Soc Lond Ser A 147:332–351
Harrington JM, Crumbliss AL (2009) The redox hypothesis in siderophore-mediated iron uptake. Biometals doi:10.1007/s10534-009-9233-4
Hersman L, Lloyd T, Sposito G (1995) Siderophore-promoted dissolution of hematite. Geochim Cosmochim Acta 59:3327–3330
Hider RC, Mohd-Nor AR, Silver J (1981) Model compounds for microbial iron-transport compounds. Part 1. Solution chemistry and Mössbauer study of iron(II) and iron(III) complexes form phenolic and catecholic systems. J Sol Chem Soc Dalton Trans 1:609–622
Hutchins DA, Bruland KW (1998) Iron-limited diatom growth and Si:N uptake ratios in a coastal upwelling regime. Nature 393:561–564
Hutchins DA, Witter AE, Butler A, Luther GW (1999) Competition among marine phytoplankton for different chelated iron species. Nature 400:858–861
Johnson KS, Gordon RM, Coale KH (1997) What controls dissolved iron concentrations in the world ocean? Mar Chem 57:137–161
Jones GJ, Palenik BP, Morel FMM (1987) Trace-metal reduction by phytoplankton-the role of plasmalemma redox enzymes. J Phycol 23:237–244
Kim D, Duckworth OW, Strathmann TJ (2009) Hydroxamate siderophore-promoted reactions between iron(II) and nitroaromatic groundwater contaminants. Geochim Cosmochim Acta 73:1297–1311
King DW, Lounsbury HA, Millero FJ (1995) Rates and mechanism of Fe(II) oxidation at nanomolar total iron concentration. Environ Sci Technol 29:818–824
Kraemer SM (2004) Iron oxide dissolution and solubility in the presence of siderophores. Aquat Sci 66:3–18
Kuma K, Nishioka J, Matsunaga K (1996) Controls on iron(III) hydroxide solubility in seawater: the influence of pH and natural organic chelators. Limnol Oceanogr 41:396–407
Liu XW, Millero FJ (2002) The solubility of iron in seawater. Mar Chem 77:43–54
Maldonado MT, Price NM (2001) Reduction and transport of organically bound iron by Thalassiosira oceanica (Bacillariophyceae). J Phycol 37:298–309
Miller WL, Kester D (1994) Photochemical iron reduction and iron bioavailability in seawater. J Mar Res 52:325–343
Millero FJ (1986) The pH of estuarine waters. Limnol Oceanogr 31:839–847
Millero FJ, Sotolongo S, Izaguirre M (1987) The oxidation kinetics of Fe(II) in seawater. Geochim Cosmochim Acta 51:793–801
Nikolić GM, Premović PI, Nicolić RS (1998) Spectrophotometric study of catechol oxidation by aerial O2 in alkaline aqueous solutions containing Mg(II) ions. Spectrosc Lett 31:327–333
Reid RT, Butler A (1991) Investigation of the mechanism of iron acquisition by the marine bacterium Alteromonas luteoviolaceus: characterization of siderophore production. Limnol Oceanogr 36:1783–1792
Rich HW, Morel FMM (1990) Availability of well-defined iron colloids to the marine diatom Thalassiosira weissflogii. Limnol Oceanogr 35:652–662
Rijkenberg MJA, Gerringa LJA, Neale PJ, Timmermans KR, Buma AGJ, de Baar HJW (2004) UVA variability overrules UVB ozone depletion effects on the photoreduction of iron in the Southern Ocean. Geophys Res Lett 31:1–5
Rijkenberg MJA, Gerringa LJA, Carolus VE, Velzeboer I, de Baar HJW (2006) Enhancement and inhibition of iron photoreduction by individual ligands in open ocean seawater. Geochim Cosmochim Acta 70:2790–2805
Rijkenberg MJA, Gerringa LJA, Timmermans KR, Fischer AC, Kroon KJ, Buma AGJ, BTh Wolterbeek, de Baar HJW (2008) Enhancement of the reactive iron pool by marine diatoms. Mar Chem 109:29–44
Santana-Casiano JM, González-Dávila M, Rodríguez MJ, Millero FJ (2000) The effect of organic compounds in the oxidation kinetics of Fe(II). Mar Chem 70:211–222
Santana-Casiano JM, González-Dávila M, Millero FJ (2005) Oxidation of nanomolar level of Fe(II) with oxygen in natural waters. Environ Sci Technol 39:2073–2079
Schweigert N, Zehnder AJB, Eggen RIL (2001) Chemical properties of catechols and their molecular modes of toxic action in cells, from microorganisms to mammals. Environ Microbiol 3:81–91
Sulzberger B, Laubscher H (1995) Reactivity of various types of iron(III) (hydr)oxides towards light-induced dissolution. Mar Chem 50:103–115
Takeda S, Kamatani A (1989) Photoreduction of Fe(III)-EDTA complex and its availability to the coastal diatom Thalassiosira weissflogii, Red Tides. Biol Environ Sci Toxicol 349–352
Theis TL, Singer PC (1974) Complexation of iron(II) by organic matter and its effect on iron(II) oxygenation. Environ Sci Technol 8:569–573
Uchimiya M, Stone AT (2006) Redox reactions between iron and quinones: thermodynamic constraints. Geochim Cosmochim Acta 70:1388–1401
Waite TD (2001) Thermodynamics of the iron system in seawater. In: Turner DR, Hunter KA (eds) The biochemistry of iron in seawater. Wiley, New York, pp 291–342
Waite TD, Morel FMM (1984) Photoreductive dissolution of colloidal iron oxide: effect of citrate. J Colloid Interface Sci 102:121–137
Wells ML (1999) Manipulating iron availability in nearshore waters. Limnol Oceanogr 44:1002–1008
Wells ML, Mayer LM (1991) The photoconversion of colloidal iron oxyhydroxides in seawater. Deep Sea Res 38:1379–1395
Wells ML, Zorkin NG, Lewis AG (1983) The role of colloid chemistry in providing a source of iron to phytoplankton. J Mar Res 41:731–746
Wells ML, Price NM, Bruland KW (1994) Iron limitation and the Cyanobacterium synechococcus in equatorial Pacific waters. Limnol Oceanogr 39:1481–1486
Wilhelm SW, Trick CG (1994) Iron-limited growth of cyanobacteria:siderophore production is a common response. Limnol Oceanogr 39:1979–1984
Winkelmann G (1991) Handbook of microbial iron chelates. CRC Press, Boca Raton
Acknowledgments
This study was supported by the Project CTM2006-09857 of Ministerio de Ciencia y Tecnología from Spain. F.J. Millero wishes to acknowledge the support of the Oceanographic Section of the National Science Foundation and the National Oceanic and Atmospheric Administration for supporting his marine physical chemistry studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Santana-Casiano, J.M., González-Dávila, M., González, A.G. et al. Fe(III) Reduction in the Presence of Catechol in Seawater. Aquat Geochem 16, 467–482 (2010). https://doi.org/10.1007/s10498-009-9088-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10498-009-9088-x