Abstract
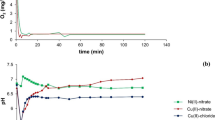
This study investigated the effects of redox-active and iron-coordinating functional groups within natural organic matter (NOM) on the electron transfer interactions between Fe(II) and 2,4-dinitrotoluene (2,4-DNT), an energetic residue often encountered in aqueous environments as a propellant component and impurities in 2,4,6-trinitrotoluene (TNT). Experiments were first conducted in homogeneous phases as a function of pH in the presence of ligands that (1) complex iron (e.g., citric acid, oxalic acid), (2) complex and reduce iron (e.g., caffeic acid, ascorbic acid), and (3) humic substances with known carboxyl content and electron transfer capacity. Then, effects of these NOM components on Fe(II) reactivity in heterogeneous media were investigated by introducing goethite. Our results indicate complex catalytic and inhibitory effects of NOM components on the reaction between Fe(II) and 2,4-DNT, depending upon the ability of NOM component to (1) reduce dissolved and particulate Fe(III) (e.g., ascorbic acid), (2) form kinetically labile dissolved Fe(II) reductants (e.g., tiron and caffeic acid), and (3) produce surface-associated Fe(II) species that are accessible to 2,4-DNT.






Similar content being viewed by others
References
Barrows SE, Cramer CJ, Truhlar DG, Elovitz MS, Weber EJ (1996) Factors controlling regioselectivity in the reduction of polynitroaromatics in aqueous solution. Environ Sci Technol 30:3028–3038
Buerge IJ, Hug SJ (1998) Influence of organic ligands on chromium(VI) reduction by iron(II). Environ Sci Technol 32:2092–2099
Chiou CT, Kile DE, Brinton TI, Malcolm RL, Leenheer JA, MacCarthy P (1987) A comparison of water solubility enhancements of organic solutes by aquatic humic materials and commercial humic acids. Environ Sci Technol 21:1231–1234
Colon D, Weber EJ, Anderson JL, Winget P, Suarez LA (2006a) Reduction of nitrosobenzenes and N-hydroxylanilines by Fe(II) species: elucidation of the reaction mechanism. Environ Sci Technol 40:4449–4454
Colon D, Weber EJ, Anderson JL (2006b) QSAR study of the reduction of nitroaromatics by Fe(II) species. Environ Sci Technol 40:4976–4982
Colon D, Weber EJ, Anderson JL (2008) Effect of natural organic matter on the reduction of nitroaromatics by Fe(II) species. Environ Sci Technol 42:6538–6543
Daun G, Lenke H, Reuss M, Knackmuss H (1998) Biological treatment of TNT-contaminated soil. 1. Anaerobic cometabolic reduction and interaction of TNT and metabolites with soil components. Environ Sci Technol 32:1956–1963
Deiana S, Gessa C, Marchetti M, Usai M (1995) Phenolic acid redox properties: pH influence on iron(III) reduction by caffeic acid. Soil Sci Soc Am J 59:1301–1307
Dunnivant FM, Schwarzenbach RP (1992) Reduction of substituted nitrobenzenes in aqueous solutions containing natural organic matter. Environ Sci Technol 26:2133–2141
Hakala JA, Chin YP, Weber EJ (2007) Influence of dissolved organic matter and Fe(II) on the abiotic reduction of pentachloronitrobenzene. Environ Sci Technol 41:7337–7342
Hofstetter TB, Heijman CG, Haderlein SB, Holliger C, Schwarzenbach RP (1999) Complete reduction of TNT and other (poly)nitroaromatic compounds under iron reducing subsurface conditions. Environ Sci Technol 33:1479–1487
Hughes JB, Wang CY, Zhang C (1999) Anaerobic biotransformation of 2, 4-dinitrotoluene and 2, 6-dinitrotoluene by Clostridium acetobutylicum: a pathway through dihydroxylamino intermediates. Environ Sci Technol 33:1065–1070
Jenkins TF, Hewitt AD, Grant CL, Thiboutot S, Ampleman G, Walsh ME, Ranney TA, Ramsey CA, Palazzo AJ, Pennington JC (2006) Identity and distribution of residues of energetic compounds at army live-fire training ranges. Chemosphere 63:1280–1290
Jones DL (1998) Organic acids in the rhizosphere—a critical review. Plant Soil 205:25–44
Klausen J, Trober SP, Haderlein SB, Schwarzenbach RP (1995) Reduction of substituted nitrobenzenes by Fe(II) in aqueous mineral suspensions. Environ Sci Technol 29:2396–2404
Larson RA, Weber EJ (1994) Reaction mechanisms in environmental organic chemistry. Lewis Publishers, Boca Raton
Linder PW, Voye A (1987) Potentiometric investigations of the equilibria between caffeic acid and copper(II), zinc(II), iron(II) and hydrogen ions in aqueous solution. Polyhedron 6:53–60
Liu D, Thomson K, Anderson AC (1984) Identification of nitroso compounds from biotransformation of 2, 4-dinitrotoluene. Appl Environ Microbiol 47:1295–1298
Marschner H (1999) Mineral nutrition of higher plants. Academic Press, London
Martell AE, Smith RM, Motekaitis RJ (2004) Critically selected stability constants of metal complexes database, US department of commerce. National Institute of Standards and Technology, Gaithersburg
Murphy EM, Zachara JM, Smith SC, Phillips JL, Wietsma TW (1994) Interaction of hydrophobic organic compounds with mineral-bound humic substances. Environ Sci Technol 28:1291–1299
Naka D, Kim D, Strathmann TJ (2006) Abiotic reduction of nitroaromatic compounds by aqueous iron(II)—catechol complexes. Environ Sci Technol 40:3006–3012
Naka D, Kim D, Carbonaro RF, Strathmann TJ (2008) Abiotic reduction of nitroaromatic contaminants by iron(II) complexes with organothiol ligands. Environ Toxicol Chem 27:1257–1266
Oh SY, Cha DK, Chiu PC (2002) Graphite-mediated reduction of 2, 4-dinitrotoluene with elemental iron. Environ Sci Technol 36:2178–2184
Peretyazhko T, Sposito G (2006) Reducing capacity of terrestrial humic acids. Geoderma 137:140–146
Rakshit S, Uchimiya M, Sposito G (2009) Iron(III) bioreduction in soil in the presence of added humic substances. Soil Sci Soc Am J 73:65–71
Raymond KN, Miller G, Matzanke BF (1984) Complexation of iron by siderophores: a review of their solution and structural chemistry and biological function. Top Curr Chem 123:49–102
Riefler RG, Smets BF (2000) Enzymatic reduction of 2, 4.6-trinitrotoluene and related nitroarenes: kinetics linked to one-electron redox potentials. Environ Sci Technol 34:3900–3906
Smolen JM, McLaughlin MA, McNevin MJ, Haberle A, Swantek S (2003) Reductive dissolution of goethite and the subsequent transformation of 4-cyanonitrobenzene: role of ascorbic acid and pH. Aquat Sci 65:308–315
Sposito G (1989) The chemistry of soils. Oxford University Press, New York
Strathmann TJ, Stone AT (2002) Reduction of oxamyl and related pesticides by FeII: influence of organic ligands and natural organic matter. Environ Sci Technol 36:5172–5183
Stumm W, Morgan JJ (1996) Aquatic chemistry. Wiley-Interscience, NY
Thorn KA, Pennington JC, Kennedy KR, Cox LG, Hayes CA, Porter BE (2008) N-15 NMR study of the immobilization of 2, 4-and 2, 6-dinitrotoluene in aerobic compost. Environ Sci Technol 42:2542–2550
Uchimiya M, Stone AT (2006) Redox reactions between iron and quinones: thermodynamic constraints. Geochim Cosmochim Acta 70:1388–1401
Uchimiya M, Stone AT (2009) Reduction of substituted p-benzoquinones by FeII at near neutral pHs. Aquat Geochem (in press)
Uchimiya M, Gorb L, Isayev O, Qasim MM, Leszczynski J (2009) One-electron standard reduction potentials of nitroaromatic and cyclic nitramine explosives. Environ Pollut (submitted)
Acknowledgments
This project was supported by the US Army Environmental Quality Technology Program at the Environmental Laboratory, US Army Engineer Research and Development Center (ERDC), Vicksburg, MS.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Uchimiya, M. Reductive Transformation of 2,4-Dinitrotoluene: Roles of Iron and Natural Organic Matter. Aquat Geochem 16, 547–562 (2010). https://doi.org/10.1007/s10498-009-9085-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10498-009-9085-0