Abstract
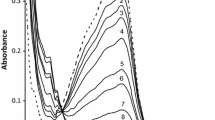
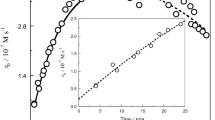
The kinetics of the formation of the purple-colored species between FeIII-EDTA and peroxynitrite were studied as a function of pH (10.4–12.3) at 22°C in aqueous solutions using a stopped-flow technique. A purple-colored species was immediately formed upon mixing, which had an absorbance maximum at 520 nm. The increase in absorbance with time could be fit empirically by a power function with two parameters a and b. The power equation determined was absorbance = a*t b, where a increased linearly with pH and the concentration of peroxynitrite, while b almost remained constant with a value of ~0.25. The molar extinction coefficient ε520 nm for the colored species was determined as 13 M−1cm−1, which is much lower than ε520 nm = 520 M−1 cm−1 for the [FeIII(EDTA)O2]3−, a purple species observed in the FeIII–EDTA–H2O2 system. The results of kinetics and spectral measurements of the present study are briefly discussed and compared with those of the reaction between Fe(III)-EDTA and hydrogen peroxide.



Similar content being viewed by others
References
Ananev V, Miklin M (2005) The peroxynitrite formation under photolysis of alkali nitrates. J Photochem Photobiol A 172:289–292
Barbeau K (2006) Photochemistry of organic iron(III) complexing ligands in oceanic systems. Photochem Photobiol 82:1505–1516
Blough NV, Zafriou OC (1985) Reaction of superoxide with nitric oxide to form peroxonitrite in alkaline aqueous solution. Inorg Chem 24:3502–3504
Bohle DS, Glassbrenner PA, Hansert B (1996) Synthesis of pure tetramethylammonium peroxynitrite. Method Enzymol 269:302–311
Brausam A, Eldik RV (2004) Further mechanistic information on the reaction between FeIIIEDTA and hydrogen peroxide: observation of second step and importance of pH. Inorg Chem 43:5351–5359
Brausam A, Maigut J, Roland M, Szilagyi PA, Buschmann HJ, Massa W, Homonnay Z, Eldik RV (2009) Detailed spectroscopic, thermodynamic, and kinetic studies on the protolytic equilibria of FeIIIcydta and the activation of hydrogen peroxide. Inorg Chem 48:7864–7884
Chu L, Anastasio C (2007) Temperature and wavelength dependence of nitrite photolysis in frozen and aqueous solutions. Environ Sci Technol 41:3626–3632
Dahl EE, Saltzman ES (2008) Alkyl nitrate photochemical production rates in North Pacific seawater. Mar Chem 112:137–141
Dahl EE, Saltzman ES, Bruyn WJD (2003) The aqueous phase yield of alkyl nitrates from ROO + NO: implications of photochemical production in seawater. Geophys Res Lett 30:1271–1274
Fan SM (2008) Photochemical and biochemical controls on reactive oxygen and iron speciation in the pelagic surface ocean. Mar Chem 109:152–164
Fischer M, Warneck P (1996) Photodecomposition of nitrite and undissociated nitrous acid in aqueous solution. J Phys Chem 100:18749–18756
Goldstein S, Czapski G (1995) The reaction of NO• with O •−2 and HO2 −: a pulse radiolysis study. Free Rad Biol Med 19:505–510
Goldstein S, Rabani J (2007) Mechanism of nitrite formation by nitrate photolysis in aqueous solutions: the role of peroxynitrite, nitrogen dioxide, and hydroxyl radical. J Am Chem Soc 129:10597–10601
Goldstein S, Lind J, Merenyi G (2005) The chemistry of peroxynitrites as compared to peroxynitrates. Chem Rev 105:2457–2470
Gustafson RL, Martell AE (1963) Hydrolytic tendencies of ferric chelates. J Phys Chem 67:576–582
Hiemstra T, Riemsdijk WHV (2006) Biogeochemical speciation of Fe in ocean water. Mar Chem 102:181–197
Huie RE, Padamaja S (1993) The reaction of nitric oxide with superoxide. Free Radical Res Commun 18:195–199
Jensen MP, Riley DP (2002) Peroxynitrite decomposition activity of iron porphyrin complexes. Inorg Chem 41:4788–4798
Kissner R, Nauser T, Bugnon P, Lye PG, Koppenol WH (1997) Formation and properties of peroxynitrite as studied by laser flash photolysis, high-pressure stopped-flow technique, and pulse radiolysis. Chem Res Toxicol 10:1285–1292
Lambert C, Soudant P, Jegaden M, Delaporte M, Labreuche Y, Moal J, Samlin JF (2007) In vitro modulation of reactive oxygen and nitrogen intermediate (ROI/RON) production in Crassostrea gigas hemocytes. Aquaculture 270:413–421
Li C, Li XZ, Graham N (2005) A study of the preparation and reactivity of potassium ferrate. Chemosphere 61:537–543
Li SX, Hong HS, Zheng FY, Deng NS (2008) Effects of metal pollution and micronutrient enrichment on the photoproduction of hydroxyl radicals in seawater by the alga Dunaliella salina. Mar Chem 108:207–214
Mack J, Bolton JR (1999) Photochemistry of nitrite and nitrate in aqueous solution: a review. J Photochem Photobiol A 128:1–13
Madsen D, Larsen J, Jensen SK, Keiding SR, Thogersen J (2003) The primary photodynamics of aqueous nitrate: formation of peroxynitrite. J Am Chem Soc 125:15571–15576
Mark G, Korth HG, Schuchmann HP, Sonntag CV (1995) The photochemistry of aqueous nitrate ion revisited. J Photochem Photobiol A 101:89–103
Morace JL (2007) Relation between selected water-quality variables, climatic factors, and lake levels in upper klamath and agency lakes, Oregon, 1990–2006. Scientific Investigation Report 2007-5117, USGS
Nauser T, Koppenol WH (2002) The rate constant of the reaction of superoxide with nitrogen monoxide: approaching the diffusion limit. J Phys Chem A 106:4084–4086
Olasehinde EF, Takeda K, Sakugawa H (2009) Development of an analytical method for nitric oxide radical determination in natural waters. Anal Chem 81:6843–6850
Richard J, Bradley E, Lanzendorf MI, Orlando TM, Hess WP (1995) Molecular NO desorption from crystalline sodium nitrate by resonant excitation of the NO3 − π–π-Transition. J Phys Chem 99:11715–11721
Rose AL, Waite TD (2005) Reduction of organically complexed ferric iron by superoxide in a simulated natural water. Environ Sci Technol 39:2645–2650
Roy EG, Wells ML, King DW (2008) Persistence of iron(II) in surface waters of the western subarctic Pacific. Limnol Oceanogr 53:89–98
Santana-Castano JM, Gonzalez-Davila M, Millero FJ (2006) The role of Fe(II) species on the oxidation of Fe(II) in natural waters in the presence of O2 and H2O2. Mar Chem 99:70–82
Sharma VK, Millero FJ, Homonnay Z (2004) Kinetics of the complex formation between iron(III)EDTA and hydrogen peroxide in aqueous solution. Inorg Chim Acta 357:3583–3586
Sharma VK, Szilgyi PA, Homonnay Z, Kuzmann E, Vertes A (2005) Mossbauer investigation of peroxo species in the iron(III)-EDTA-H2O2 system. Eur J Chem 1186–1189
Shimanovich R, Groves JT (2001) Mechanism of peroxynitrite decomposition catalyzed by FeTMPS a bioactive sulfonated iron porphyrin. Arch Biochem Biophys 387:307–317
Torrilles J, Romestand B (2001) In vitro production of peroxynitrite by haemocytes from marine bivalves: C0ELISA determination of 3-nitrotyrosine level in plasma proteins from Mytilus galloprovincialis and Crassostrea gigas. BMC Immunol 2:1–6
Treinin A, Hayon E (1970) Absorption spectra and reaction kinetics of NO2, N2O3, and N2O4 in aqueous solution. J Am Chem Soc 92:5821–5828
Ward BB, Zafiriou OC (1988) Nitrification and nitric oxide in the oxygen minimum of the eastern tropical North Pacific. Deep-Sea Res 35:1127–1142
Warneck P, Wuzinger C (1988) Product quantum yields for the 305-nm photodecomposition of NO3 − in aqueous solution. J Phys Chem 92:6278–6283
Acknowledgment
We wish to thank Dr. Mary Sohn for useful comments on the paper. We also thank reviewers for their useful comments to improve the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, V.K., Yngard, R.A., Homonnay, Z. et al. The Kinetics of the Interaction Between Iron(III)-Ethylenediaminetetraacetate and Peroxynitrite. Aquat Geochem 16, 483–490 (2010). https://doi.org/10.1007/s10498-009-9083-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10498-009-9083-2