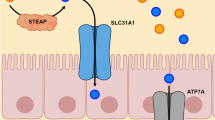
Abstract
Lung cancer (LC) is a serious threat to mankind. The survival of LC patients is still poor despite the enormous efforts that have been made to develop novel treatments. A copper-dependent cell death termed cuproptosis is distinct from known programmed cell death (PCD). Cuproptosis is induced by the disruption of the binding of copper to lipoylated tricarboxylic acid (TCA) cycle proteins of mitochondrial respiratory chains. Potential approaches for treating LC are inducing cell cuproptosis and targeting cell copper death mechanisms. Thus, in this review, we summarize the systemic and cellular metabolic processes of copper. We highlight the possible therapeutic options of employing copper ionophores and chelators for inducing cuproptosis. Moreover, we summarize the prognostic models based on cuproptosis-related genes (CRGs) to identify promising biomarkers for tumor diagnosis and therapy. This review aims to provide a comprehensive summary of CRGs-based prognostic models and promising therapeutic options for cuproptosis induction in LC.

Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- ALDH2:
-
Aldehyde dehydrogenase 2
- ATP7A:
-
ATPase copper transporting alpha
- ATP7B:
-
ATPase copper transporting beta
- BP:
-
Biological processes
- MP:
-
Molecular processes
- CDKN2A:
-
Cyclin-dependent kinase inhibitor 2 A
- CRGs:
-
Cuproptosis-related genes
- CuO-NPs:
-
Copper oxide nanoparticles
- DLAT:
-
Drolipoamide S-acetyltransferase
- DLD:
-
Dihydrolipoamide dehydrogenase
- FDX1:
-
Ferredoxin 1
- GEO:
-
Gene expression omnibus
- GLS:
-
Glutaminase
- GO:
-
Gene ontology
- GTEx:
-
Genotype-tissue expression
- HIF-1:
-
Hypoxia-inducible factor-1
- IL-8:
-
Interleukin-8
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- LC:
-
Lung cancer
- LIAS:
-
Lipoic acid synthetase
- LIPT1:
-
Lipoyltransferase 1
- LOX:
-
Lysyl oxidase
- MTF1:
-
Metal regulatory transcription factor 1
- PCD:
-
Programmed cell death
- PDC:
-
Pyruvate dehydrogenase complex
- PDHA1:
-
Pyruvate dehydrogenase E1 subunit alpha 1
- PDHB:
-
Pyruvate dehydrogenase E1 subunit beta
- PD-L1:
-
Programmed death ligand 1
- PLGA:
-
Poly lactic-co-glycolic acid
- ROS:
-
Reactive oxygen species
- SLC31A1:
-
Solute carrier family 31 member 1
- TCA:
-
Tricarboxylic acid
- TCGA:
-
The cancer genome atlas database
- UCSC:
-
University of california santa cruz
- VEGF:
-
Vascular endothelial growth factor
References
Siegel RL, Miller KD, Fuchs HE, Jemal A (2021) Cancer statistics, 2021. CA Cancer J Clin 71(1):7–33
Ruiz-Cordero R, Devine WP (2020) Targeted therapy and checkpoint immunotherapy in Lung Cancer. Surg Pathol Clin 13(1):17–33
Xia C, Dong X, Li H, Cao M, Sun D, He S et al (2022) Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl) 135(5):584–590
Miller YE (2005) Pathogenesis of lung cancer: 100 year report. Am J Respir Cell Mol Biol 33(3):216–223
Wang Y, Huang J, Wu Q, Zhang J, Ma Z, Ma S et al (2021) Downregulation of breast cancer resistance protein by long-term fractionated radiotherapy sensitizes lung adenocarcinoma to SN-38. Invest New Drugs 39(2):458–468
Osmani L, Askin F, Gabrielson E, Li QK (2018) Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): moving from targeted therapy to immunotherapy. Semin Cancer Biol 52(Pt 1):103–109
Testa U, Castelli G, Pelosi E (2018) Lung cancers: molecular characterization, clonal heterogeneity and evolution, and Cancer Stem cells. Cancers (Basel) 10(8)
Ali A, Goffin JR, Arnold A, Ellis PM (2013) Survival of patients with non-small-cell lung cancer after a diagnosis of brain metastases. Curr Oncol 20(4):e300–e306
Howlader N, Noone AM, Krapcho M SEER cancer statistics review, 1975–2017
Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G (2019) The molecular machinery of regulated cell death. Cell Res 29(5):347–364
Tsvetkov P, Coy S, Petrova B, Dreishpoon M, Verma A, Abdusamad M et al (2022) Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 375(6586):1254–1261
Ge EJ, Bush AI, Casini A, Cobine PA, Cross JR, DeNicola GM et al (2022) Connecting copper and cancer: from transition metal signalling to metalloplasia. Nat Rev Cancer 22(2):102–113
Capriotti G, Piccardo A, Giovannelli E, Signore A (2022) Targeting copper in Cancer Imaging and Therapy: a New Theragnostic Agent. J Clin Med. ;12(1)
Bost M, Houdart S, Oberli M, Kalonji E, Huneau JF, Margaritis I (2016) Dietary copper and human health: current evidence and unresolved issues. J Trace Elem Med Biol 35:107–115
Lonnerdal B (2008) Intestinal regulation of copper homeostasis: a developmental perspective. Am J Clin Nutr 88(3):846S–50S
Hernandez S, Tsuchiya Y, Garcia-Ruiz JP, Lalioti V, Nielsen S, Cassio D et al (2008) ATP7B copper-regulated traffic and association with the tight junctions: copper excretion into the bile. Gastroenterology 134(4):1215–1223
Zhang X, Yang Q (2018) Association between serum copper levels and lung cancer risk: a meta-analysis. J Int Med Res 46(12):4863–4873
Salvador F, Martin A, Lopez-Menendez C, Moreno-Bueno G, Santos V, Vazquez-Naharro A et al (2017) Lysyl oxidase-like protein LOXL2 promotes lung metastasis of breast Cancer. Cancer Res 77(21):5846–5859
Su Y, Zhang X, Li S, Xie W, Guo J (2022) Emerging roles of the Copper-CTR1 Axis in Tumorigenesis. Mol Cancer Res 20(9):1339–1353
Yu Z, Zhou R, Zhao Y, Pan Y, Liang H, Zhang JS et al (2019) Blockage of SLC31A1-dependent copper absorption increases pancreatic cancer cell autophagy to resist cell death. Cell Prolif 52(2):e12568
Yun Y, Wang Y, Yang E, Jing X (2022) Cuproptosis-related gene - SLC31A1, FDX1 and ATP7B - polymorphisms are Associated with risk of Lung Cancer. Pharmgenomics Pers Med 15:733–742
Zhu X, Boulet A, Buckley KM, Phillips CB, Gammon MG, Oldfather LE et al (2021) Mitochondrial copper and phosphate transporter specificity was defined early in the evolution of eukaryotes. Elife. ;10
La Fontaine S, Ackland ML, Mercer JF (2010) Mammalian copper-transporting P-type ATPases, ATP7A and ATP7B: emerging roles. Int J Biochem Cell Biol 42(2):206–209
Grasso M, Bond GJ, Kim YJ, Boyd S, Matson Dzebo M, Valenzuela S et al (2021) The copper chaperone CCS facilitates copper binding to MEK1/2 to promote kinase activation. J Biol Chem 297(6):101314
Hu Q, Wang R, Ma H, Zhang Z, Xue Q (2022) Cuproptosis predicts the risk and clinical outcomes of lung adenocarcinoma. Front Oncol 12:922332
Liu Y, Lin W, Yang Y, Shao J, Zhao H, Wang G et al (2022) Role of cuproptosis-related gene in lung adenocarcinoma. Front Oncol 12:1080985
Sun X, Li Z, Meng F, Huang X, Wang J, Song J et al (2022) Cuproptosis associated genes affect prognosis and tumor microenvironment infiltration characterization in lung adenocarcinoma. Am J Cancer Res 12(10):4545–4565
Wang S, Xing N, Meng X, Xiang L, Zhang Y (2022) Comprehensive bioinformatics analysis to identify a novel cuproptosis-related prognostic signature and its ceRNA regulatory axis and candidate traditional Chinese medicine active ingredients in lung adenocarcinoma. Front Pharmacol 13:971867
Zhang H, Shi Y, Yi Q, Wang C, Xia Q, Zhang Y et al (2022) A novel defined cuproptosis-related gene signature for predicting the prognosis of lung adenocarcinoma. Front Genet 13:975185
Zhang W, Qu H, Ma X, Li L, Wei Y, Wang Y et al (2023) Identification of cuproptosis and immune-related gene prognostic signature in lung adenocarcinoma. Front Immunol 14:1179742
Patel MS, Nemeria NS, Furey W, Jordan F (2014) The pyruvate dehydrogenase complexes: structure-based function and regulation. J Biol Chem 289(24):16615–16623
Zheng P, Zhou C, Lu L, Liu B, Ding Y (2022) Elesclomol: a copper ionophore targeting mitochondrial metabolism for cancer therapy. J Exp Clin Cancer Res 41(1):271
Jiao Y, Hannafon BN, Ding WQ (2016) Disulfiram’s anticancer activity: evidence and mechanisms. Anticancer Agents Med Chem 16(11):1378–1384
Wangpaichitr M, Wu C, You M, Maher JC, Dinh V, Feun LG et al (2009) N’,N’-Dimethyl-N’,N’-bis(phenylcarbonothioyl) propanedihydrazide (Elesclomol) selectively kills cisplatin resistant lung Cancer cells through reactive oxygen species (ROS). Cancers (Basel) 1(1):23–38
Wang W, Wang J, Liu S, Ren Y, Wang J, Liu S et al (2022) An EHMT2/NFYA-ALDH2 signaling axis modulates the RAF pathway to regulate paclitaxel resistance in lung cancer. Mol Cancer 21(1):106
Nechushtan H, Hamamreh Y, Nidal S, Gotfried M, Baron A, Shalev YI et al (2015) A phase IIb trial assessing the addition of disulfiram to chemotherapy for the treatment of metastatic non-small cell lung cancer. Oncologist 20(4):366–367
Wang C, Yang J, Han H, Chen J, Wang Y, Li Q et al (2017) Disulfiram-loaded porous PLGA microparticle for inhibiting the proliferation and migration of non-small-cell lung cancer. Int J Nanomed 12:827–837
Lowndes SA, Harris AL (2005) The role of copper in tumour angiogenesis. J Mammary Gland Biol Neoplasia 10:299–310
Salvo J, Sandoval C (2022) Role of copper nanoparticles in wound healing for chronic wounds: literature review. Burns Trauma. ;10
Sciegienka SJ, Solst SR, Falls KC, Schoenfeld JD, Klinger AR, Ross NL et al (2017) D-penicillamine combined with inhibitors of hydroperoxide metabolism enhances lung and breast cancer cell responses to radiation and carboplatin via H(2)O(2)-mediated oxidative stress. Free Radic Biol Med 108:354–361
Singh J, Kaur G, Rawat M (2016) A brief review on synthesis and characterization of copper oxide nanoparticles and its applications. J Bioelectron Nanotechnol. ;1(9)
Meghana S, Kabra P, Chakraborty S, Padmavathy N (2015) Understanding the pathway of antibacterial activity of copper oxide nanoparticles. RSC Adv 5(16):12293–12299
Zhao H, Maruthupandy M, Al-mekhlafi FA, Chackaravarthi G, Ramachandran G, Chelliah CK (2022) Biological synthesis of copper oxide nanoparticles using marine endophytic actinomycetes and evaluation of biofilm producing bacteria and A549 lung cancer cells. J King Saud Univ - Sci 34(3):101866
Voli F, Valli E, Lerra L, Kimpton K, Saletta F, Giorgi FM et al (2020) Intratumoral Copper modulates PD-L1 expression and influences Tumor Immune Evasion. Cancer Res 80(19):4129–4144
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
RJ: Conceptualization, writing, and illustrating. HB: Review and editing. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jawed, R., Bhatti, H. Cuproptosis in lung cancer: therapeutic options and prognostic models. Apoptosis (2024). https://doi.org/10.1007/s10495-024-01978-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s10495-024-01978-x