Abstract
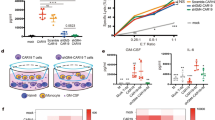
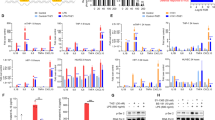
Cytokine release syndrome (CRS) is a great challenge for the application of anti-CD19 CAR-T cell therapy. The aim of this study was to investigate the effect of knocking down interferon gamma (IFN-γ) by shRNA as a potential strategy to reduce the cytokine storms. A newly designed short hairpin interference RNA of IFN-γ (shIFN-γ) in CD19CAR gene was constructed. Several cellular model systems of approach using Nalm-6 cell lines including Nalm-6CD19pos and Nalm-6CD19neg with or without monocytes and endothelial cells were used to analyze the different levels of cytokines after shIFN-γ-anti-CD19CAR-T cell targeted therapy. The activity of this novel CD19CAR-T was evaluated both in vitro and in NSG mouse model. The killing efficacy of shIFN-γ-anti-CD19CAR-T at the E:T ratio of 2:1 was similar to that of regular anti-CD19CAR-T at the E:T ratio of 1:1. The IFN-γ level in the shIFN-γ-anti-CD19CAR-T cell group was (2673.1 ± 307.4) pg/ml at the E:T ratio of 2:1 which was significantly lower than that ((8261.5 ± 345.5) pg/ml) in the regular anti-CD19CAR-T group at the E:T ratio of 1:1. Cytotoxicity experiments in vitro showed significantly reduced concentrations of IFN-γ, IL-6 and TNFα in the shIFN-γ-anti-CD19CAR-T cell group compared to regular anti-CD19CAR-T cell group. Both regular anti-CD19CAR and shIFN-γ-CD19CAR-T exerted bystander killing effect in vitro. We conclude that shIFN-γ-anti-CD19CAR-T cells can reduce the generation of cytokine storms without significantly compromising their therapeutic efficacy in the preclinical setting. In mouse model, 3 × 106 shIFN-γ-anti-CD19CAR-T cells/mouse generated the similar killing efficacy to that with 2 × 106 regular anti-CD19CAR-T cells/mouse.





Similar content being viewed by others
Data Availability
Data will be made available on request.
Abbreviations
- CRS:
-
Cytokine release syndrome
- IFN-γ:
-
Interferon‐gamma
- shIFN-γ:
-
short hairpin interference RNA of IFN-γ
- CAR-T:
-
Chimeric antigen receptor T cells
- R/R B-ALL:
-
relapsed/refractory B-lineage acute lymphoblastic leukemia
- IL-6:
-
interleukin 6
References
Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H et al (2018) Tisagenlecleucel in Children and Young adults with B-Cell lymphoblastic Leukemia. N Engl J Med 378(5):439–448
Levine JE, Grupp SA, Pulsipher MA, Dietz AC, Rives S, Myers GD et al (2021) Pooled safety analysis of tisagenlecleucel in children and young adults with B cell acute lymphoblastic Leukemia. J Immunother Cancer. ;9(8)
Curran KJ, Margossian SP, Kernan NA, Silverman LB, Williams DA, Shukla N et al (2019) Toxicity and response after CD19-specific CAR T-cell therapy in pediatric/young adult relapsed/refractory B-ALL. Blood 134(26):2361–2368
Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M et al (2018) Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med 24(6):739–748
Tvedt THA, Vo AK, Bruserud Ø, Reikvam H (2021) Cytokine release syndrome in the Immunotherapy of Hematological malignancies: the Biology behind and possible clinical consequences. J Clin Med. ;10(21)
Agrawal N, Dasaradhi PV, Mohmmed A, Malhotra P, Bhatnagar RK, Mukherjee SK (2003) RNA interference: biology, mechanism, and applications. Microbiol Mol Biology Reviews: MMBR 67(4):657–685
de Bruyns A, Geiling B, Dankort D (2016) Construction of modular lentiviral vectors for effective gene expression and knockdown. Methods in molecular biology. (Clifton NJ) 1448:3–21
Yang X, Wu X, Yang Y, Gu T, Hong L, Zheng E et al (2019) Improvement of developmental competence of cloned male pig embryos by short hairpin ribonucleic acid (shRNA) vector-based but not small interfering RNA (siRNA)-mediated RNA interference (RNAi) of xist expression. J Reprod Dev 65(6):533–539
Lambeth LS, Smith CA (2013) Short hairpin RNA-mediated gene silencing. Methods in molecular biology. (Clifton NJ) 942:205–232
Kang L, Tang X, Zhang J, Li M, Xu N, Qi W et al (2020) Interleukin-6-knockdown of chimeric antigen receptor-modified T cells significantly reduces IL-6 release from monocytes. Experimental Hematol Oncol 9:11
Ba D, Li H, Liu R, Zhang P, Tang Y (2023) Exploratory study on the efficacy of bortezomib combining mitoxantrone or CD22-CAR T therapy targeting CD19-negative relapse after CD19-CAR T cell therapy with a simpler cell-line-based model. Apoptosis: An International Journal on Programmed cell Death.
Nicholson IC, Lenton KA, Little DJ, Decorso T, Lee FT, Scott AM et al (1997) Construction and characterisation of a functional CD19 specific single chain fv fragment for immunotherapy of B lineage Leukaemia and Lymphoma. Mol Immunol 34(16–17):1157–1165
Tang Y, Xu X, Song H, Yang S, Shi S, Wei J et al (2008) Early diagnostic and prognostic significance of a specific Th1/Th2 cytokine pattern in children with haemophagocytic syndrome. Br J Haematol 143(1):84–91
Read JA, Rouce RH, Mo F, Mamonkin M, King KY (2023) Apoptosis of hematopoietic stem cells contributes to bone marrow suppression following Chimeric Antigen Receptor T Cell Therapy. Transp Cell Therapy 29(3):165.e1-e7
Teachey DT, Lacey SF, Shaw PA, Melenhorst JJ, Maude SL, Frey N et al (2016) Identification of predictive biomarkers for Cytokine Release Syndrome after chimeric Antigen receptor T-cell therapy for Acute Lymphoblastic Leukemia. Cancer Discov 6(6):664–679
Hay KA, Hanafi LA, Li D, Gust J, Liles WC, Wurfel MM et al (2017) Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood 130(21):2295–2306
Darragh LB, Karam SD (2022) Amateur antigen-presenting cells in the Tumor microenvironment. Mol Carcinog 61(2):153–164
Bailey SR, Vatsa S, Larson RC, Bouffard AA, Scarfò I, Kann MC et al (2022) Blockade or deletion of IFNγ reduces macrophage activation without compromising CAR T-cell function in hematologic malignancies. Blood cancer Discovery 3(2):136–153
Alizadeh D, Wong RA, Gholamin S, Maker M, Aftabizadeh M, Yang X et al (2021) IFNγ is critical for CAR T cell-mediated myeloid activation and induction of endogenous immunity. Cancer Discov 11(9):2248–2265
Kale SD, Mehrkens BN, Stegman MM, Kastelberg B, Carnes H, McNeill RJ et al (2020) Small intestinal immunopathology plays a big role in Lethal Cytokine Release Syndrome, and its modulation by Interferon-γ, IL-17A, and a Janus kinase inhibitor. Front Immunol 11:1311
Kozma GT, Mészáros T, Bakos T, Hennies M, Bencze D, Uzonyi B et al (2021) Mini-factor H modulates complement-dependent IL-6 and IL-10 release in an Immune Cell Culture (PBMC) Model: potential benefits against Cytokine Storm. Front Immunol 12:642860
Parker BS, Rautela J, Hertzog PJ (2016) Antitumour actions of interferons: implications for cancer therapy. Nat Rev Cancer 16(3):131–144
Raskov H, Orhan A, Christensen JP, Gögenur I (2021) Cytotoxic CD8(+) T cells in cancer and cancer immunotherapy. Br J Cancer 124(2):359–367
Zhang H, Lv X, Kong Q, Tan Y (2022) IL-6/IFN-γ double knockdown CAR-T cells reduce the release of multiple cytokines from PBMCs in vitro. Hum Vaccines Immunotherapeutics 18(1):1–14
Liu D, Zhao J (2018) Cytokine release syndrome: grading, modeling, and new therapy. J Hematol Oncol 11(1):121
Kearney CJ, Vervoort SJ, Hogg SJ, Ramsbottom KM, Freeman AJ, Lalaoui N et al (2018) Tumor immune evasion arises through loss of TNF sensitivity. Sci Immunol 3(23)
Groom JR, Luster AD (2011) CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol 89(2):207–215
Thibaut R, Bost P, Milo I, Cazaux M, Lemaître F, Garcia Z et al (2020) Bystander IFN-γ activity promotes widespread and sustained cytokine signaling altering the Tumor microenvironment. Nat cancer 1(3):302–314
Giavridis T, van der Stegen SJC, Eyquem J, Hamieh M, Piersigilli A, Sadelain M (2018) CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med 24(6):731–738
Ye C, Yang H, Cheng M, Shultz LD, Greiner DL, Brehm MA et al (2020) A rapid, sensitive, and reproducible in vivo PBMC humanized murine model for determining therapeutic-related cytokine release syndrome. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology 34(9):12963–12975
Acknowledgements
Authors would like to thank all the staffs from the department/center of hematology-oncology and laboratory for their technical support.
Funding
This work was supported in part by grants from the National Natural Science Foundation of China (grant no. 81770202 and 81470304), Zhejiang Medical and Health Science and Technology Plan Project (No. 2022KY868), the Key Project from Science and Technology Department of Zhejiang Province (No. 2019C03032), Pediatric Leukemia Diagnostic and Therapeutic Technology Research Center of Zhejiang Province (No. JBZX-201904), Zhejiang Medical and Health Science and Technology Plan Project (No. 2022KY1051) and the Scientific Research Foundation of Zhejiang University City College (J-202103).
Author information
Authors and Affiliations
Contributions
P.Z. and P.Y. are co-first authors of this article who did experiments and performed data analysis. P.Z. wrote the manuscript draft. Y.T., H.L. and N.Z. did part of experiments. R.L .and S.L. assisted in completing the statistical analysis. Y.T. designed the work and amended the manuscript. Y.T. assisted in amending the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional of animal care and use committee, ZJCLA. (ID :ZJCLA-IACUC-20030115)
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, P., Ying, P., Li, H. et al. A novel safer CD19CAR with shRNA interference of IFN-γ can reduce multiple cytokine levels without significantly compromising its killing efficacy. Apoptosis 29, 556–567 (2024). https://doi.org/10.1007/s10495-023-01925-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-023-01925-2