Abstract
Purpose
We aimed to evaluate whether pulmonary fibrosis occurs in type 2 diabetes rat models and whether VD3 can prevent it by inhibiting pyroptosis.
Methods
Sprague-Dawley rats were assigned to normal control (NC), diabetic model control (MC), low-dose VD3 (LVD), medium-dose VD3 (MVD), high-dose VD3 (HVD) and metformin positive control (PC) groups. Type 2 diabetes model was induced by a high-sugar, high-fat diet combined with STZ injection, and subsequently intervened with VD3 or metformin for 10 weeks. Blood glucose, body weight, food intake, water intake, urine volume, morphology, lung hydroxyproline level, immunohistochemistry, TUNEL staining, inflammatory cytokines secretion and related protein expression were analyzed.
Results
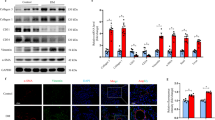
Diabetic rats exhibited significant impairments in fasting blood glucose, insulin resistance, body weight, food intake, water intake, and urine volume. While morphological parameters, diabetic rats exhibited severe lung fibrosis. Intriguingly, VD3 intervention reversed, at least in part, the diabetes-induced alterations. The expression of pyroptosis-related proteins was up-regulated in diabetic lungs whereas the changes were reversed by VD3. In the meanwhile, SIRT3 expression was down-regulated in diabetic lungs while VD3 up-regulated it.
Conclusion
Fibrotic changes were observed in diabetic rat lung tissue and our study indicates that VD3 may effectively ameliorate diabetic pulmonary fibrosis via SIRT3-mediated suppression of pyroptosis.






Similar content being viewed by others
Data Availability
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
Association AD (2018) 4. Lifestyle Management. Diabetes care 41, S38-S50. https://doi.org/10.2337/dc18-S004
Guariguata L, Whiting DR, Hambleton I et al (2014) Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes research and clinical practice. 103:137–149. https://doi.org/10.1016/j.diabres.2013.11.002
Bloomgarden ZT (2005) Diabetic nephropathy. Diabetes Care 28:745–751
Cheung N, Mitchell PWong TY (2010) Diabetic retinopathy. Lancet (London, England) 376, 124–136. https://doi.org/10.1016/S0140-6736(09)62124-3
Pitocco D, Fuso L, Conte EG et al (2012) The diabetic lung–a new target organ? The review of diabetic studies: RDS. 9:23–35. https://doi.org/10.1900/RDS.2012.9.23
Kuitert LME (2008) The lung in diabetes–yet another target organ? Chronic respiratory disease 5. 67–68. https://doi.org/10.1177/1479972308091408
Caner B, Ugur O, Bayraktar M et al (1994) Impaired lung epithelial permeability in diabetics detected by technetium-99m-DTPA aerosol scintigraphy. J nuclear medicine: official publication Soc Nuclear Med 35:204–206
Tuleta IFrangogiannis N G (2021) Diabetic fibrosis. Biochimica et biophysica acta molecular basis of disease 1867. 166044. https://doi.org/10.1016/j.bbadis.2020.166044
Morgan M, JLiu Z G Crosstalk of reactive oxygen species and NF-κB signaling
Lawrence T The nuclear factor NF-kappaB pathway in inflammation
Mack M (2018) Inflammation and fibrosis. Matrix Biology: Journal of the International Society for Matrix Biology 68–69. 106–121. https://doi.org/10.1016/j.matbio.2017.11.010
Chen Y, Zhang F, Wang D et al (2020) Mesenchymal Stem Cells Attenuate Diabetic Lung Fibrosis via Adjusting Sirt3-Mediated Stress Responses in Rats. Oxidative Medicine and Cellular Longevity 2020, 8076105. https://doi.org/10.1155/2020/8076105
Bause A, SHaigis M, C (2013) SIRT3 regulation of mitochondrial oxidative stress. Exp Gerontol 48:634–639. https://doi.org/10.1016/j.exger.2012.08.007
Dikalova AE, Pandey A, Xiao L et al (2020) Mitochondrial deacetylase Sirt3 reduces vascular dysfunction and hypertension while Sirt3 depletion in essential hypertension is linked to vascular inflammation and oxidative stress. Circul Res 126:439–452. https://doi.org/10.1161/CIRCRESAHA.119.315767
Chen C, Gu J, Wang J et al (2021) Physcion 8-O-β-glucopyranoside ameliorates liver fibrosis through inflammation inhibition by regulating SIRT3-mediated NF-κB P65 nuclear expression. Int Immunopharmacol 90:107206. https://doi.org/10.1016/j.intimp.2020.107206
Lv D, Luo M, Yan J et al (2021) Protective effect of Sirtuin 3 on CLP-Induced endothelial dysfunction of early Sepsis by inhibiting NF-κB and NLRP3 signaling pathways. Inflammation 44:1782–1792. https://doi.org/10.1007/s10753-021-01454-7
Wu J, Jin ZYan LJ (2017) Redox imbalance and mitochondrial abnormalities in the diabetic lung. Redox Biol 11:51–59. https://doi.org/10.1016/j.redox.2016.11.003
Giulietti A, van Etten E, Overbergh L et al (2007) Monocytes from type 2 diabetic patients have a pro-inflammatory profile. 1,25-Dihydroxyvitamin D(3) works as anti-inflammatory. Diabetes Res Clin Pract 77:47–57
Wang H, Zhang Q, Chai Y et al (2015) 1,25(OH)2D3 downregulates the toll-like receptor 4-mediated inflammatory pathway and ameliorates liver injury in diabetic rats. J Endocrinol Investig 38:1083–1091. https://doi.org/10.1007/s40618-015-0287-6
Ahmad S, Arora S, Khan S et al (2021) Vitamin D and its therapeutic relevance in pulmonary diseases. J Nutr Biochem 90:108571. https://doi.org/10.1016/j.jnutbio.2020.108571
Lecube A, Simó R, Pallayova M et al (2017) Pulmonary function and sleep breathing: two new targets for type 2 Diabetes Care. Endocr Rev 38:550–573. https://doi.org/10.1210/er.2017-00173
van den Borst B, Gosker HR, Zeegers MP et al (2010) Pulmonary function in diabetes: a metaanalysis. Chest 138:393–406. https://doi.org/10.1378/chest.09-2622
Ehrlich SF, Quesenberry CP, Van Den Eeden SK et al (2010) Patients diagnosed with diabetes are at increased risk for asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, and pneumonia but not lung cancer. Diabetes Care 33:55–60. https://doi.org/10.2337/dc09-0880
Tecilazich F, Formenti A, MGiustina A (2021) Role of vitamin D in diabetic retinopathy: pathophysiological and clinical aspects. Reviews In Endocrine & Metabolic Disorders 22:715–727. https://doi.org/10.1007/s11154-020-09575-4
Karonova T, Stepanova A, Bystrova A et al (2020) High-dose vitamin D supplementation improves Microcirculation and reduces inflammation in Diabetic Neuropathy Patients. Nutrients 12 https://doi.org/10.3390/nu12092518
Qu H, Lin K, Wang H et al (2017) 1,25(OH) D improves cardiac dysfunction, hypertrophy, and fibrosis through PARP1/SIRT1/mTOR-related mechanisms in type 1 diabetes. 61. Molecular nutrition & food researchhttps://doi.org/10.1002/mnfr.201600338
Båvenholm PN, Pigon J, Ostenson CG et al (2001) Insulin sensitivity of suppression of endogenous glucose production is the single most important determinant of glucose tolerance. Diabetes 50:1449–1454
Bai Y, Zang X, Ma J et al (2016) Anti-diabetic effect of Portulaca oleracea L. Polysaccharideandits mechanism in Diabetic rats. Int J Mol Sci 17. https://doi.org/10.3390/ijms17081201
Zhang D, Meng HYang H-s (2012) Antidiabetic activity of Taxus cuspidata polysaccharides in streptozotocin-induced diabetic mice. Int J Biol Macromol 50:720–724. https://doi.org/10.1016/j.ijbiomac.2011.12.020
Xiong W-T, Gu L, Wang C et al (2013) Anti-hyperglycemic and hypolipidemic effects of Cistanche tubulosa in type 2 diabetic db/db mice. J Ethnopharmacol 150:935–945. https://doi.org/10.1016/j.jep.2013.09.027
Pan L, Li Z, Wang Y et al (2020) Network pharmacology and metabolomics study on the intervention of traditional chinese medicine Huanglian Decoction in rats with type 2 diabetes mellitus. J Ethnopharmacol 258:112842. https://doi.org/10.1016/j.jep.2020.112842
Pitocco D, Zaccardi F, Di Stasio E et al (2010) Oxidative stress, nitric oxide, and diabetes. The review of diabetic studies: RDS. 7:15–25. https://doi.org/10.1900/RDS.2010.7.15
Niroomand M, Fotouhi A, Irannejad N et al (2019) Does high-dose vitamin D supplementation impact insulin resistance and risk of development of diabetes in patients with pre-diabetes? A double-blind randomized clinical trial. Diabetes Res Clin Pract 148:1–9. https://doi.org/10.1016/j.diabres.2018.12.008
Davidson MB, Duran P, Lee ML et al (2013) High-dose vitamin D supplementation in people with prediabetes and hypovitaminosis D. Diabetes care. 36:260–266. https://doi.org/10.2337/dc12-1204
Ban C, RTwigg S M (2008) Fibrosis in diabetes complications: pathogenic mechanisms and circulating and urinary markers. Vasc Health Risk Manag 4:575–596
Lee S, BKalluri R (2010) Mechanistic connection between inflammation and fibrosis. Kidney International Supplement. https://doi.org/10.1038/ki.2010.418. S22-S26
Wynn T, ARamalingam T, R (2012) Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 18:1028–1040. https://doi.org/10.1038/nm.2807
Lamouille S, Xu JDerynck R (2014) Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 15:178–196. https://doi.org/10.1038/nrm3758
Yu M-A, Shin K-S, Kim JH et al (2009) HGF and BMP-7 ameliorate high glucose-induced epithelial-to-mesenchymal transition of peritoneal mesothelium. J Am Soc Nephrology: JASN 20:567–581. https://doi.org/10.1681/ASN.2008040424
Nieto MA, Huang RY-J, Jackson RA et al (2016) EMT: 2016. Cell 166:21–45. https://doi.org/10.1016/j.cell.2016.06.028
Hill C, Jones MG, Davies DE et al (2019) Epithelial-mesenchymal transition contributes to pulmonary fibrosis via aberrant epithelial/fibroblastic cross-talk. J Lung Health Dis 3:31–35
Salton F, Volpe M, CConfalonieri M (2019) Epithelial Mesenchymal transition in the Pathogenesis of Idiopathic Pulmonary Fibrosis. Medicina (Kaunas. Lithuania) 55. https://doi.org/10.3390/medicina55040083
Nathan SD, Brown A, WKing C S (2016) Pathogenesis of idiopathic pulmonary fibrosis. Guide to Clinical Management of Idiopathic Pulmonary Fibrosis. Springer International Publishing, Cham, pp 43–51
Wilson M, SWynn TA (2009) Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol 2:103–121. https://doi.org/10.1038/mi.2008.85
Shi J, Gao WShao F (2017) Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends. Biochem Sci 42:245–254. https://doi.org/10.1016/j.tibs.2016.10.004
Frank DVince JE (2019) Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death and Differentiation 26. https://doi.org/10.1038/s41418-018-0212-6
Song M, Wang J, Sun Y et al (2022) Inhibition of gasdermin D-dependent pyroptosis attenuates the progression of silica-induced pulmonary inflammation and fibrosis. Acta Pharm Sinica B 12:1213–1224. https://doi.org/10.1016/j.apsb.2021.10.006
Zhao Q, Hao C, Wei J et al (2021) Bone marrow-derived mesenchymal stem cells attenuate silica-induced pulmonary fibrosis by inhibiting apoptosis and pyroptosis but not autophagy in rats. Ecotoxicol Environ Saf 216:112181. https://doi.org/10.1016/j.ecoenv.2021.112181
Liang Q, Cai W, Zhao Y et al (2020) Lycorine ameliorates bleomycin-induced pulmonary fibrosis via inhibiting NLRP3 inflammasome activation and pyroptosis. Pharmacol Res 158:104884. https://doi.org/10.1016/j.phrs.2020.104884
Gao J, Peng S, Shan X et al (2019) Inhibition of AIM2 inflammasome-mediated pyroptosis by Andrographolide contributes to amelioration of radiation-induced lung inflammation and fibrosis. Cell Death Dis 10:957. https://doi.org/10.1038/s41419-019-2195-8
Perico L, Morigi MBenigni A (2016) Mitochondrial sirtuin 3 and renal Diseases. Nephron 134:14–19. https://doi.org/10.1159/000444370
Sosulski ML, Gongora R, Feghali-Bostwick C et al (2017) Sirtuin 3 Deregulation promotes pulmonary fibrosis. The journals of Gerontology Series A, Biological Sciences and Medical Sciences. 72:595–602. https://doi.org/10.1093/gerona/glw151
Chen C-J, Fu Y-C, Yu W et al (2013) SIRT3 protects cardiomyocytes from oxidative stress-mediated cell death by activating NF-κB. Biochemical and biophysical research communications. 430:798–803. https://doi.org/10.1016/j.bbrc.2012.11.066
Tyagi A, Nguyen CU, Chong T et al (2018) SIRT3 deficiency-induced mitochondrial dysfunction and inflammasome formation in the brain. Sci Rep 8:17547. https://doi.org/10.1038/s41598-018-35890-7
Kurundkar D, Kurundkar AR, Bone NB et al (2019) SIRT3 diminishes inflammation and mitigates endotoxin-induced acute lung injury. JCI Insight 4. https://doi.org/10.1172/jci.insight.120722
Funding
This work was supported by the National Natural Science Foundation of China, grant number (82173515, 82003454, 81872626).
Author information
Authors and Affiliations
Contributions
Substantial contributions to the conception or design of the work: Lu-Lu Tang, Xing Li. Substantial contributions to the acquisition, analysis or interpretation of data for the work: Lu-Lu Tang, Xing Li and Dong-Dong Zhang. Drafting the work or revising it critically for important intellectual content: Lu-Lu Tang, Xing Li, Dong-Dong Zhang, Yu-Jing Zhang, Yang-Yang Peng, Meng-Xin Li, Han-Lu Song, Hao Chen and Wen-Jie Li. Final approval of the version to be published: Lu-Lu Tang, Xing Li, Dong-Dong Zhang, Yu-Jing Zhang, Yang-Yang Peng, Meng-Xin Li, Han-Lu Song, Hao Chen and Wen-Jie Li.
Corresponding author
Ethics declarations
Competing interests
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
All experiments involving animal subjects were performed in accordance with guidelines approved by the Animal Care and Use Committee of Zhengzhou University (ZZUGZR2018-035).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, L., Zhang, D., Zhang, Y. et al. Vitamin D3 alleviates lung fibrosis of type 2 diabetic rats via SIRT3 mediated suppression of pyroptosis. Apoptosis 28, 1618–1627 (2023). https://doi.org/10.1007/s10495-023-01878-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-023-01878-6