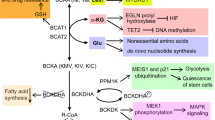
Abstract
Amino acids (AAs) are crucial molecules for the synthesis of mammalian proteins as well as a source of energy and redox equilibrium maintenance. The development of tumors also requires AAs as nutrients. Increased AAs metabolism is frequently seen in tumor cells to produce enough biomass, energy, and reduction agents. However, increased AA demand may result in auxotrophy in some cancer cells, highlighting the vulnerabilities of cancers and exposing the AA metabolism as a potential target for cancer therapy. The dynamic balance of cell survival and death is required for cellular homeostasis, growth, and development. Malignant cells manage to avoid cell death through a range of mechanisms, such as developing an addiction to amino acids through metabolic adaptation. In order to offer some guidance for AA-targeted cancer therapy, we have outlined the function of AA metabolism in tumor progression, the modalities of cell death, and the regulation of AA metabolism on tumor cell death in this review.




Similar content being viewed by others
Data availability
Not applicable.
References
Agirre X et al (2003) TP53 is frequently altered by methylation, mutation, and/or deletion in acute lymphoblastic leukaemia. Mol Carcinog 38(4):201–208
Akram M (2014) Citric acid cycle and role of its intermediates in metabolism. Cell Biochem Biophys 68(3):475–478
Amelio I et al (2014) Serine and glycine metabolism in cancer. Trends Biochem Sci 39(4):191–198
Ananieva EA, Wilkinson AC (2018) Branched-chain amino acid metabolism in cancer. Curr Opin Clin Nutr Metab Care 21(1):64–70
Audia JE, Campbell RM (2016) Histone modifications and cancer. Cold Spring Harb Perspect Biol 8(4):a019521
Bean GR et al (2016) A metabolic synthetic lethal strategy with arginine deprivation and chloroquine leads to cell death in ASS1-deficient sarcomas. Cell Death Dis 7(10):e2406
Berger SL et al (2009) An operational definition of epigenetics. Genes Dev 23(7):781–783
Bersuker K et al (2019) The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575(7784):688–692
Bodineau C et al (2022) Glutamine, MTOR and autophagy: a multiconnection relationship. Autophagy 18(11):2749–2750
Broer S, Gauthier-Coles G (2022) Amino acid homeostasis in mammalian cells with a focus on amino acid transport. J Nutr 152(1):16–28
Brosnan JT, Brosnan ME (2013) Glutamate: a truly functional amino acid. Amino Acids 45(3):413–418
Chen MS et al (2017) CHAC1 degradation of glutathione enhances cystine-starvation-induced necroptosis and ferroptosis in human triple negative breast cancer cells via the GCN2-eIF2alpha-ATF4 pathway. Oncotarget 8(70):114588–114602
Chmelarova M et al (2013) Methylation in the p53 promoter in epithelial ovarian cancer. Clin Transl Oncol 15(2):160–163
Chung WJ et al (2005) Inhibition of cystine uptake disrupts the growth of primary brain tumors. J Neurosci 25(31):7101–7110
Cluntun AA et al (2017) Glutamine metabolism in cancer: understanding the heterogeneity. Trends Cancer 3(3):169–180
Cools J (2012) Improvements in the survival of children and adolescents with acute lymphoblastic leukemia. Haematologica 97(5):635
Currie E et al (2013) Cellular fatty acid metabolism and cancer. Cell Metab 18(2):153–161
Da LDR et al (2018) Leucine reduces the proliferation of MC3T3-E1 cells through DNA damage and cell senescence. Toxicol In Vitro 48:1–10
Dai X et al (2021) Programmed cell death, redox imbalance, and cancer therapeutics. Apoptosis 26(7–8):385–414
Dawson MA (2012) Cancer epigenetics: from mechanism to therapy. Cell 150(1):12–27
Diaz-Vivancos P et al (2015) Glutathione–linking cell proliferation to oxidative stress. Free Radic Biol Med 89:1154–1164
Dixon SJ et al (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149(5):1060–1072
Edwards JR et al (2017) DNA methylation and DNA methyltransferases. Epigenet Chromatin 10:23
Ekici S et al (2022) Glutamine imaging: a new avenue for glioma management. AJNR Am J Neuroradiol 43(1):11–18
Ellis L et al (2009) Epigenetics in cancer: targeting chromatin modifications. Mol Cancer Ther 8(6):1409–1420
Endicott M et al (2021) Amino acid metabolism as a therapeutic target in cancer: a review. Amino Acids 53(8):1169–1179
Falcone M et al (2022) Sensitisation of cancer cells to radiotherapy by serine and glycine starvation. Br J Cancer 127(10):1773–1786
Fan J et al (2014) Quantitative flux analysis reveals folate-dependent NADPH production. Nature 510(7504):298–302
Fan K et al (2022) Targeting nutrient dependency in cancer treatment. Front Oncol 12:820173
Frank D, Vince JE (2019) Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Differ 26(1):99–114
Fuchs BC, Bode BP (2006) Stressing out over survival: glutamine as an apoptotic modulator. J Surg Res 131(1):26–40
Galluzzi L, Kroemer G (2008) Necroptosis: a specialized pathway of programmed necrosis. Cell 135(7):1161–1163
Galluzzi L et al (2018) Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 25(3):486–541
Gao M et al (2022) Understanding the mechanistic regulation of ferroptosis in cancer: the gene matters. J Genet Genomics 49(10):913–926
Glick D et al (2010) Autophagy: cellular and molecular mechanisms. J Pathol 221(1):3–12
Gopisetty G et al (2006) DNA methylation and apoptosis. Mol Immunol 43(11):1729–1740
Green CR et al (2016) Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat Chem Biol 12(1):15–21
Hanaki S, Shimada M (2021) Targeting EZH2 as cancer therapy. J Biochem 170(1):1–4
Hoffer LJ (2016) Human protein and amino acid requirements. JPEN J Parenter Enteral Nutr 40(4):460–474
Jahani M et al (2018) Arginine: challenges and opportunities of this two-faced molecule in cancer therapy. Biomed Pharmacother 102:594–601
Jaramillo MC, Zhang DD (2013) The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev 27(20):2179–2191
Ji Y et al (2017) Deprivation of asparagine triggers cytoprotective autophagy in laryngeal squamous cell carcinoma. Appl Microbiol Biotechnol 101(12):4951–4961
Jiang J et al (2021) Asparagine: a metabolite to be targeted in cancers. Metabolites 11(6):402
Jiang YJ et al (2021) Excessive ROS production and enhanced autophagy contribute to myocardial injury induced by branched-chain amino acids: roles for the AMPK-ULK1 signaling pathway and alpha7nAChR. Biochim Biophys Acta Mol Basis Dis 1867(1):165980
Ju HQ et al (2020) NADPH homeostasis in cancer: functions, mechanisms and therapeutic implications. Signal Transduct Target Ther 5(1):231
Kaiser WJ, Sridharan H et al (2013) Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem 288(43):31268–31279
Kamei Y et al (2020) Regulation of skeletal muscle function by amino acids. Nutrients 12(1):261
Khalil N, Abi-Habib RJ (2020) [HuArgI (co)-PEG5000]-induced arginine deprivation leads to autophagy dependent cell death in pancreatic cancer cells. Invest New Drugs 38(5):1236–1246
Klaunig JE (2018) Oxidative stress and cancer. Curr Pharm Des 24(40):4771–4778
Kovacs SB, Miao EA (2017) Gasdermins: effectors of pyroptosis. Trends Cell Biol 27(9):673–684
Le X et al (2020) DNA methylation downregulated ZDHHC1 suppresses tumor growth by altering cellular metabolism and inducing oxidative/ER stress-mediated apoptosis and pyroptosis. Theranostics 10(21):9495–9511
Lee MG et al (2006) Promoter CpG hypermethylation and downregulation of XAF1 expression in human urogenital malignancies: implication for attenuated p53 response to apoptotic stresses. Oncogene 25(42):5807–5822
Lewis CA et al (2014) Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of mammalian cells. Mol Cell 55(2):253–263
Li Z, Zhang H (2016) Reprogramming of glucose, fatty acid and amino acid metabolism for cancer progression. Cell Mol Life Sci 73(2):377–392
Lieu EL et al (2020) Amino acids in cancer. Exp Mol Med 52(1):15–30
Lin CC et al (2020) RIPK3 upregulation confers robust proliferation and collateral cystine-dependence on breast cancer recurrence. Cell Death Differ 27(7):2234–2247
Ling H et al (2022) Glycine increased ferroptosis via SAM-mediated GPX4 promoter methylation in rheumatoid arthritis. Rheumatology (Oxford) 61(11):4521–4534
Liu Y, Levine B (2015) Autosis and autophagic cell death: the dark side of autophagy. Cell Death Differ 22(3):367–376
Lu SC (2013) Glutathione synthesis. Biochim Biophys Acta 1830(5):3143–3153
Maddocks O et al (2017) Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature 544(7650):372–376
Majumdar R et al (2016) Glutamate, ornithine, arginine, proline, and polyamine metabolic interactions: the pathway is regulated at the post-transcriptional level. Front Plant Sci 7:78
Mates JM et al (2006) Pathways from glutamine to apoptosis. Front Biosci 11:3164–3180
Mayers JR, Wu C et al (2014) Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat Med 20(10):1193–1198
Metallo CM et al (2011) Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 481(7381):380–384
Michalak EM et al (2019) The roles of DNA, RNA and histone methylation in ageing and cancer. Nat Rev Mol Cell Biol 20(10):573–589
Mitsuishi Y et al (2012) Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell 22(1):66–79
Moffatt BA, Ashihara H (2002) Purine and pyrimidine nucleotide synthesis and metabolism. Arabidopsis Book 1:e0018
Moreno-Sanchez R et al (2017) Control of the NADPH supply for oxidative stress handling in cancer cells. Free Radic Biol Med 112:149–161
Neinast M et al (2019) Branched chain amino acids. Annu Rev Physiol 81:139–164
Newman AC, Maddocks O (2017) One-carbon metabolism in cancer. Br J Cancer 116(12):1499–1504
Nikiforov MA et al (2002) A functional screen for Myc-responsive genes reveals serine hydroxymethyltransferase, a major source of the one-carbon unit for cell metabolism. Mol Cell Biol 22(16):5793–5800
Ohtawa K et al (1998) Apoptosis of leukemia cells induced by valine-deficient medium. Leukemia 12(10):1651–1652
Parzych KR, Klionsky DJ (2014) An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal 20(3):460–473
Pavlova NN et al (2018) As extracellular glutamine levels decline, asparagine becomes an essential amino acid. Cell Metab 27(2):428-438.e5
Peng F et al (2022) Regulated cell death (RCD) in cancer: key pathways and targeted therapies. Signal Transduct Target Ther 7(1):286
Pentimalli F et al (2019) Cell death pathologies: targeting death pathways and the immune system for cancer therapy. Genes Immun 20(7):539–554
Poillet-Perez L et al (2018) Autophagy maintains tumour growth through circulating arginine. Nature 563(7732):569–573
Purohit V et al (2019) Metabolic regulation of redox balance in cancer. Cancers (Basel) 11(7):955
Qi Y et al (2017) Fluorine-18 labeled amino acids for tumor PET/CT imaging. Oncotarget 8(36):60581–60588
Sedillo JC, Cryns VL (2022) Targeting the methionine addiction of cancer. Am J Cancer Res 12(5):2249–2276
Sheen JH et al (2011) Defective regulation of autophagy upon leucine deprivation reveals a targetable liability of human melanoma cells in vitro and in vivo. Cancer Cell 19(5):613–628
Shi J et al (2017) Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem Sci 42(4):245–254
Shimizu S et al (2014) Autophagic cell death and cancer. Int J Mol Sci 15(2):3145–3153
Shuvalov O et al (2017) One-carbon metabolism and nucleotide biosynthesis as attractive targets for anticancer therapy. Oncotarget 8(14):23955–23977
Shuvayeva GY et al (2021) Indospicine combined with arginine deprivation triggers cancer cell death via caspase-dependent apoptosis. Cell Biol Int 45(3):518–527
Shyh-Chang N et al (2013) Influence of threonine metabolism on S-adenosylmethionine and histone methylation. Science 339(6116):222–226
Son SM et al (2019) Leucine signals to mTORC1 via its metabolite acetyl-coenzyme A. Cell Metab 29(1):192-201.e7
Song P et al (2015) Asparaginase induces apoptosis and cytoprotective autophagy in chronic myeloid leukemia cells. Oncotarget 6(6):3861–3873
Sun RC, Denko NC (2014) Hypoxic regulation of glutamine metabolism through HIF1 and SIAH2 supports lipid synthesis that is necessary for tumor growth. Cell Metab 19(2):285–292
Suzuki A, Iwata J (2021) Amino acid metabolism and autophagy in skeletal development and homeostasis. Bone 146:115881
Tajan M et al (2021) Serine synthesis pathway inhibition cooperates with dietary serine and glycine limitation for cancer therapy. Nat Commun 12(1):366
Vandenabeele P et al (2010) Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 11(10):700–714
Vettore L et al (2020) New aspects of amino acid metabolism in cancer. Br J Cancer 122(2):150–156
Villa E, Ricci JE (2016) How does metabolism affect cell death in cancer? FEBS J 283(14):2653–2660
Vynnytska BO et al (2011) Canavanine augments proapoptotic effects of arginine deprivation in cultured human cancer cells. Anticancer Drugs 22(2):148–157
Wanders D et al (2020) Methionine restriction and cancer biology. Nutrients 12(3):684
Wang Y et al (2021) The double-edged roles of ROS in cancer prevention and therapy. Theranostics 11(10):4839–4857
Wheatley DN (2004) Controlling cancer by restricting arginine availability-arginine- catabolizing enzymes as anticancer agents. Anticancer Drugs 15(9):825–833
White PJ et al (2021) Insulin action, type 2 diabetes, and branched-chain amino acids: a two-way street. Mol Metab 52:101261
Wilder CS, Chen Z et al (2022) Pharmacologic approaches to amino acid depletion for cancer therapy. Mol Carcinog 61(2):127–152
Wong CC et al (2016) SLC25A22 promotes proliferation and survival of colorectal cancer cells With KRAS mutations and xenograft tumor progression in mice via intracellular synthesis of aspartate. Gastroenterology 151(5):945-960.e6
Xiao F et al (2016) Leucine deprivation inhibits proliferation and induces apoptosis of human breast cancer cells via fatty acid synthase. Oncotarget 7(39):63679–63689
Yang L et al (2017) Glutaminolysis: a hallmark of cancer metabolism. Annu Rev Biomed Eng 19:163–194
Yang WS et al (2014) Regulation of ferroptotic cancer cell death by GPX4. Cell 156(1–2):317–331
Ying H et al (2012) Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 149(3):656–670
Yoo HC, Han JM (2022) Amino acid metabolism in cancer drug resistance. Cells 11(1):140
Zhang J et al (2014) Asparagine plays a critical role in regulating cellular adaptation to glutamine depletion. Mol Cell 56(2):205–218
Zhang X et al (2020) Homocysteine induces oxidative stress and ferroptosis of nucleus pulposus via enhancing methylation of GPX4. Free Radic Biol Med 160:552–565
Zhang Y et al (2008) Structural biology of the purine biosynthetic pathway. Cell Mol Life Sci 65(23):3699–3724
Funding
The Key Research and Development Program of Shaanxi, China (No. 2020SF-061 to LZ).
Author information
Authors and Affiliations
Contributions
JW conceived and wrote this manuscript. HW and MG drawn the figures in this manuscript. YZ revised the manuscript according to the reviewer’s comments. LZ provided funding support, KT and DH provided revised suggestions for this manuscript. QX, and KT supervised the manuscript. All authors listed in this manuscript have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, J., Wang, H., Gao, M. et al. The regulation of amino acid metabolism in tumor cell death: from the perspective of physiological functions. Apoptosis 28, 1304–1314 (2023). https://doi.org/10.1007/s10495-023-01875-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-023-01875-9