Abstract
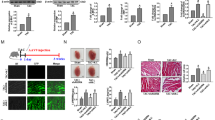
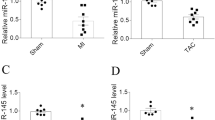
Abnormal levels of CHI3L1 and lnc TUG1 are often associated with myocardial fibrosis, and their specific expressions may be closely related to the process of myocardial fibrosis. In addition, CHI3L1 was found to significantly up-regulate the expression of lncTUG1. Therefore, this study further explored the major role of CHI3L1 in regulating the progression of myocardial fibrosis. Myocardial fibrosis in mice was established using an angiotensin (Ang II) model, and the degree of myocardial fibrosis was assessed by qPCR, western blot and pathological techniques. HL-1 cells with overexpression and silencing of CHI3L1 were constructed, and the cell migration ability was detected using the Transwell method. Biological information was used to predict the potential target miRNA of lnc TUG1, and the interaction between them was verified by dual luciferase reporter assay. Using functional rescue assay and the rAAV9 technique, CHI3L1 was verified to affect the fibrotic process of myocardial cells by regulating the lnc TUG1/miR-495-3p/ETS1 axis in vitro and in vivo. The myocardial fibrosis index in the model group was significantly upregulated, and expression of both CHI3L1 and lnc TUG1 was upregulated. Pathological results revealed fibrosis and collagen deposition in the myocardium. Overexpression of lnc TUG1 reversed the inhibitory effect of CHI3L1 silencing on myocardial fibrosis. Mechanistically, CH3L1 upregulates the expression of lnc TUG1, and lnc TUG1 weakens the inhibition of ETS1 through sponge absorption of miR-495-3p, promoting the process of myocardial fibrosis.








Similar content being viewed by others
Data Availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Kim S, Iwao H (2000) Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacol Rev 52:11–34
Wynn TA (2008) Cellular and molecular mechanisms of fibrosis. J Pathol 214:199–210. https://doi.org/10.1002/path.2277
Meagher PB, Lee XA, Lee J, Visram A, Friedberg MK, Connelly KA (2021) Cardiac Fibrosis: key role of integrins in Cardiac Homeostasis and Remodeling. https://doi.org/10.3390/cells10040770
Johansen JS, Williamson MK, Rice JS, Price PA (1992) Identification of proteins secreted by human osteoblastic cells in culture. J Bone Miner Res 7:501–512. https://doi.org/10.1002/jbmr.5650070506
Yeo IJ, Lee CK, Han SB, Yun J, Hong JT (2019) Roles of chitinase 3-like 1 in the development of cancer, neurodegenerative diseases, and inflammatory diseases. Pharmacol Ther 203:107394. https://doi.org/10.1016/j.pharmthera.2019.107394
Wang Q, Shen H, Min J et al (2018) YKL-40 is highly expressed in the epicardial adipose tissue of patients with atrial fibrillation and associated with atrial fibrosis. J Transl Med 16:229. https://doi.org/10.1186/s12967-018-1598-0
Kornblit B, Hellemann D, Munthe-Fog L et al (2013) Plasma YKL-40 and CHI3L1 in systemic inflammation and sepsis-experience from two prospective cohorts. Immunobiology 218:1227–1234. https://doi.org/10.1016/j.imbio.2013.04.010
Johansen JS (2006) Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan Med Bull 53:172–209
Sebastiani G, Alberti A (2006) Non invasive fibrosis biomarkers reduce but not substitute the need for liver biopsy. World J Gastroenterol 12:3682–3694. https://doi.org/10.3748/wjg.v12.i23.3682
Pizano-Martínez O, Yañez-Sánchez I, Alatorre-Carranza P et al (2011) YKL-40 expression in CD14+ liver cells in acute and chronic injury. World J Gastroenterol 17:3830–3835. https://doi.org/10.3748/wjg.v17.i33.3830
Archer K, Broskova Z, Bayoumi AS et al (2015) Long non-coding RNAs as Master regulators in Cardiovascular Diseases. Int J Mol Sci 16:23651–23667. https://doi.org/10.3390/ijms161023651
Schonrock N, Harvey RP, Mattick JS (2012) Long noncoding RNAs in cardiac development and pathophysiology. Circ Res 111:1349–1362. https://doi.org/10.1161/circresaha.112.268953
Zhu Y, Feng Z, Jian Z, Xiao Y (2018) Long noncoding RNA TUG1 promotes cardiac fibroblast transformation to myofibroblasts via miR–29c in chronic hypoxia. Mol Med Rep 18:3451–3460. https://doi.org/10.3892/mmr.2018.9327
Abd El-Fattah AA, Sadik NAH, Shaker OG, Mohamed A, Kamal (2018) Single nucleotide polymorphism in SMAD7 and CHI3L1 and colorectal Cancer risk. https://doi.org/10.1155/2018/9853192. Mediators Inflamm 2018:9853192.
Yuan X, Pan J, Wen L et al (2019) MiR-144-3p enhances Cardiac Fibrosis after myocardial infarction by Targeting PTEN. Front Cell Dev Biol 7:249. https://doi.org/10.3389/fcell.2019.00249
Sopel MJ, Rosin NL, Lee TD, Légaré JF (2011) Myocardial fibrosis in response to angiotensin II is preceded by the recruitment of mesenchymal progenitor cells. Lab Invest 91:565–578. https://doi.org/10.1038/labinvest.2010.190
Gao J, Guo Y, Chen Y, Zhou J, Liu Y, Su P (2019) Adeno-associated virus 9-mediated RNA interference targeting SOCS3 alleviates diastolic heart failure in rats. Gene 697:11–18. https://doi.org/10.1016/j.gene.2019.01.044
Yang L, Dong H, Lu H et al (2019) Serum YKL-40 predicts long-term outcome in patients undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Med (Baltim) 98e14920. https://doi.org/10.1097/md.0000000000014920
Canpolat U, Aytemir K, Hazirolan T, Özer N, Oto A (2015) Serum YKL-40 as a marker of Left Atrial Fibrosis assessed by delayed enhancement MRI in Lone Atrial Fibrillation. Pacing Clin Electrophysiol 38:1386–1395. https://doi.org/10.1111/pace.12729
Vainio LE, Szabó Z, Lin R et al (2019) Connective tissue growth factor inhibition enhances Cardiac repair and limits Fibrosis after myocardial infarction. JACC Basic Transl Sci 4:83–94. https://doi.org/10.1016/j.jacbts.2018.10.007
Dean RG, Balding LC, Candido R et al (2005) Connective tissue growth factor and cardiac fibrosis after myocardial infarction. J Histochem Cytochem 53:1245–1256. https://doi.org/10.1369/jhc.4A6560.2005
Pedram A, Razandi M, O’Mahony F, Lubahn D, Levin ER (2010) Estrogen receptor-beta prevents cardiac fibrosis. Mol Endocrinol 24:2152–2165. https://doi.org/10.1210/me.2010-0154Epub 2010 Sep 1
Ma X, Zhao A, Yao Y et al (2015) Therapeutic delivery of cyclin-A2 via recombinant adeno-associated virus serotype 9 restarts the myocardial cell cycle: an in vitro study. Mol Med Rep 11:3652–3658. https://doi.org/10.3892/mmr.2015.3147
Wang X, Jin H, Jiang S, Xu Y (2018) MicroRNA-495 inhibits the high glucose-induced inflammation, differentiation and extracellular matrix accumulation of cardiac fibroblasts through downregulation of NOD1. Cell Mol Biol Lett 23:23. https://doi.org/10.1186/s11658-018-0089-x
Gong L, Wu X, Li X et al (2020) S1PR3 deficiency alleviates radiation-induced pulmonary fibrosis through the regulation of epithelial-mesenchymal transition by targeting miR-495-3p. J Cell Physiol 235:2310–2324. https://doi.org/10.1002/jcp.29138
Hahne JC, Okuducu AF, Sahin A, Fafeur V, Kiriakidis S, Wernert N (2008) The transcription factor ETS-1: its role in tumour development and strategies for its inhibition. Mini Rev Med Chem 8:1095–1105. https://doi.org/10.2174/138955708785909934
Xu L, Fu M, Chen D et al (2019) Endothelial-specific deletion of Ets-1 attenuates angiotensin II-induced cardiac fibrosis via suppression of endothelial-to-mesenchymal transition. BMB Rep 52:595–600. https://doi.org/10.5483/BMBRep.2019.52.10.206
Hao G, Han Z, Meng Z et al (2015) Ets-1 upregulation mediates angiotensin II-related cardiac fibrosis. Int J Clin Exp Pathol 8:10216–10227
Kumar U, Hu Y, Masrour N et al (2021) MicroRNA-495/TGF-β/FOXC1 axis regulates multidrug resistance in metaplastic breast cancer cells. Biochem Pharmacol 192:114692. https://doi.org/10.1016/j.bcp.2021.114692
Czuwara-Ladykowska J, Sementchenko VI, Watson DK, Trojanowska M (2002) Ets1 is an effector of the transforming growth factor beta (TGF-beta) signaling pathway and an antagonist of the profibrotic effects of TGF-beta. J Biol Chem 277:20399–20408. https://doi.org/10.1074/jbc.M200206200
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
YP S, XG L and DS M carried out the studies, participated in collecting data, and drafted the manuscript. X S and JT G performed the statistical analysis and participated in its design. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
All the authors declare that they have no conflict of interest.
Ethics approval and consent to participate
All animal experiments conform to the standards of the Animal Ethics Committee. This study was approved by the ethics committee of The First Hospital of Jilin University (SY20191012).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, Y., Shan, X., Guo, J. et al. CHI3L1 promotes myocardial fibrosis via regulating lncRNA TUG1/miR-495-3p/ETS1 axis. Apoptosis 28, 1436–1451 (2023). https://doi.org/10.1007/s10495-023-01859-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-023-01859-9