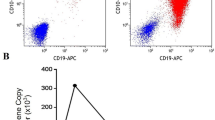
Abstract
Target-negative relapse after CD19 chimeric antigen receptor engineered (CAR) T cell therapy for patients with B lineage acute lymphoblastic leukemia (B-ALL) presents limited treatment options with dismal outcomes. Although CD22-CAR T cells mediate similarly potent antineoplastic effects in patients with CD19dim or even CD19-negative relapse following CD19-directed immunotherapy, a high rate of relapse associated with diminished CD22 cell surface expression has also been observed. Therefore, it is unclear whether any other therapeutic options are available. Mitoxantrone has shown significant antineoplastic activity in patients with relapsed or refractory leukemia over the past decades, and in some cases, the addition of bortezomib to conventional chemotherapeutic agents has demonstrated improved response rates. However, whether this mitoxantrone and bortezomib combination therapy is effective for those patients who have relapsed B-ALL after receiving CD19-CAR T cell therapy remains to be elucidated. In this study, we established a cellular model system using a CD19-positive B-ALL cell line Nalm-6 to investigate the treatment options for CD19-negative relapsed B-ALL after CD19-CAR T cell therapy. In addition to CD22-CAR T therapy, we observed that the combination of bortezomib and mitoxantrone exhibited effective anti-leukemia activity in the CD19-negative Nalm-6 cell line by downregulating p-AKT and p-mTOR. These results suggest that this combination therapy is a possible option for target-negative refractory leukemia cells after CAR-T cell treatment.






Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Curran KJ, Margossian SP, Kernan NA et al (2019) Toxicity and response after CD19-specific CAR T-cell therapy in pediatric/young adult relapsed/refractory B-ALL.Blood(. 26:2361–2368. https://doi.org/10.1182/blood.2019001641
Fry TJ, Shah NN, Orentas RJ et al (2018) CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy.Nat Med. 1:20–28. https://doi.org/10.1038/nm.4441
Lee DW, Kochenderfer JN, Stetler-Stevenson M et al (2015) T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial.Lancet(. 9967:517–528. https://doi.org/10.1016/s0140-6736(14)61403-3
Gardner R, Wu D, Cherian S et al (2016) Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy.Blood(. 20:2406. https://doi.org/10.1182/blood-2015-08-665547
Majzner RG (2018) Mackall.Tumor Antigen escape from CAR T-cell Therapy.Cancer Discov. 10:1219–1226. https://doi.org/10.1158/2159-8290.CD-18-0442
Liu S, Deng B, Yin Z et al (2021) Combination of CD19 and CD22 CAR-T cell therapy in relapsed B-cell acute lymphoblastic leukemia after allogeneic transplantation. Am J Hematol 6:671–679. https://doi.org/10.1002/ajh.26160
Guedan S, Delgado.Immobilizing J, Moving Target A (2019) CAR T cells hit CD22.Clin Cancer Res. 17:5188–5190. https://doi.org/10.1158/1078-0432.CCR-19-1649
Raponi S, De Propris MS, Intoppa S et al (2011) Flow cytometric study of potential target antigens (CD19, CD20, CD22, CD33) for antibody-based immunotherapy in acute lymphoblastic leukemia: analysis of 552 cases.Leuk lymphoma. 6:1098–1107. https://doi.org/10.3109/10428194.2011.559668
Pan J, Niu Q, Deng B et al (2019) CD22 CAR T-cell therapy in refractory or relapsed B acute lymphoblastic leukemia.Leukemia(. 12:2854–2866. https://doi.org/10.1038/s41375-019-0488-7
Fousek K, Watanabe J, Joseph SK et al (2021) CAR T-cells that target acute B-lineage leukemia irrespective of CD19 expression.Leukemia(. 1:75–89. https://doi.org/10.1038/s41375-020-0792-2
Martyniszyn A, Krahl AC, Andre MC et al (2017) CD20-CD19 bispecific CAR T cells for the treatment of B-Cell Malignancies.Hum Gene Ther. 12:1147–1157. https://doi.org/10.1089/hum.2017.126
Qin H, Ramakrishna S, Nguyen S et al (2018) Preclinical Development of Bivalent chimeric Antigen Receptors Targeting both CD19 and CD22.Mol Ther Oncolytics. 127–137. https://doi.org/10.1016/j.omto.2018.10.006
Hegde M, Mukherjee M, Grada Z et al (2016) Tandem CAR T cells targeting HER2 and IL13Ralpha2 mitigate tumor antigen escape. J Clin Invest 8:3036–3052. https://doi.org/10.1172/JCI83416
Liedtke M, Dunn T, Dinner S et al (2014) Salvage therapy with mitoxantrone, etoposide and cytarabine in relapsed or refractory acute lymphoblastic leukemia. Leuk Res 12:1441–1445. https://doi.org/10.1016/j.leukres.2014.09.018
Mitsiades N, Mitsiades CS, Richardson PG et al (2003) The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic applications.Blood(. 6:2377–2380. https://doi.org/10.1182/blood-2002-06-1768
Horton TM, Perentesis JP, Gamis AS et al (2014) A phase 2 study of bortezomib combined with either idarubicin/cytarabine or cytarabine/etoposide in children with relapsed, refractory or secondary acute myeloid leukemia: a report from the Children’s Oncology Group.Pediatr Blood Cancer. 10:1754–1760. https://doi.org/10.1002/pbc.25117
Horton TM, Pati D, Plon SE et al (2007) A phase 1 study of the proteasome inhibitor bortezomib in pediatric patients with refractory leukemia: a Children’s Oncology Group study.Clin Cancer Res. 5:1516–1522. https://doi.org/10.1158/1078-0432.CCR-06-2173
Horton TM, Whitlock JA, Lu X et al (2019) Bortezomib reinduction chemotherapy in high-risk ALL in first relapse: a report from the Children’s Oncology Group. Br J Haematol 2:274–285. https://doi.org/10.1111/bjh.15919
La Starza R, Cambò B, Pierini A et al (2019) Venetoclax and Bortezomib in Relapsed/Refractory Early T-Cell Precursor Acute Lymphoblastic Leukemia. https://doi.org/10.1200/po.19.00172. JCO Precis Oncol.(
Schneider D, Xiong Y, Wu D et al (2017) A tandem CD19/CD20 CAR lentiviral vector drives on-target and off-target antigen modulation in leukemia cell lines. J Immunother Cancer. https://doi.org/10.1186/s40425-017-0246-1
Schachterle W, Badwe CR, Palikuqi B et al (2017) Sox17 drives functional engraftment of endothelium converted from non-vascular cells. Nat Commun. https://doi.org/10.1038/ncomms13963
Wang P, Huang Z, Peng Y et al, Circular RNA et al circBNC2 inhibits epithelial cell G2-M arrest to prevent fibrotic maladaptive repair.Nat Commun.(2022)1:6502.doi: https://doi.org/10.1038/s41467-022-34287-5
Wang Z, Wang Y, Wang S et al Coxsackievirus A6 induces cell cycle arrest in G0/G1 phase for viral production.Front Cell Infect Microbiol.(2018)279.doi: https://doi.org/10.3389/fcimb.2018.00279
Jiang SX, Qi B, Yao WJ et al (2017) Berberine displays antitumor activity in esophageal cancer cells in vitro. World J Gastroenterol 14:2511–2518. https://doi.org/10.3748/wjg.v23.i14.2511
Yang F, Zhang J, Zhang X et al (2019) Delayed remission following sequential infusion of humanized CD19- and CD22-modified CAR-T cells in a patient with relapsed/refractory acute lymphoblastic leukemia and prior exposure to murine-derived CD19-directed CAR-T cells.Onco targets Ther. 2187–2191. https://doi.org/10.2147/OTT.S189103
Lee DW, Stetler-Stevenson M, Yuan CM et al (2016) Long-term outcomes following CD19 CAR T cell therapy for B-ALL are Superior in Patients receiving a Fludarabine/Cyclophosphamide preparative regimen and Post-CAR hematopoietic. Stem Cell Transplantation Blood 22:218–218. https://doi.org/10.1182/blood.V128.22.218.218
Skorka K, Ostapinska K, Malesa A et al The application of CAR-T cells in Haematological Malignancies.Arch Immunol Ther Exp (Warsz).(2020)6:34.doi: https://doi.org/10.1007/s00005-020-00599-x
Köhl U, Arsenieva S, Holzinger A et al (2018) CAR T cells in trials: recent Achievements and Challenges that remain in the production of modified T cells for clinical Applications.Hum Gene Ther. 5:559–568. https://doi.org/10.1089/hum.2017.254
Holstein SA, Lunning MA (2020) .CAR T-Cell therapy in hematologic malignancies: a voyage. Progress Clin Pharmacol Ther 1:112–122. https://doi.org/10.1002/cpt.1674
Abreu TR, Fonseca NA, Gonçalves N et al (2020) Current challenges and emerging opportunities of CAR-T cell therapies.J Control Release. 246–261. https://doi.org/10.1016/j.jconrel.2019.12.047
Shouse G, Danilov AV, Artz A (2022) .CAR T-Cell therapy in the older person: indications and Risks.Curr Oncol Rep. 9:1189–1199. https://doi.org/10.1007/s11912-022-01272-6
Dinner S, Lee D (2014) Liedtke.Current therapy and novel agents for relapsed or refractory acute lymphoblastic leukemia.Leuk lymphoma. 8:1715–1724. https://doi.org/10.3109/10428194.2013.856428
Bertaina A, Vinti L, Strocchio L et al (2017) The combination of bortezomib with chemotherapy to treat relapsed/refractory acute lymphoblastic leukaemia of childhood. Br J Haematol 4:629–636. https://doi.org/10.1111/bjh.14505
Story JY, Zoine JT, Burnham RE et al (2021) Bortezomib enhances cytotoxicity of ex vivo-expanded gamma delta T cells against acute myeloid leukemia and T-cell acute lymphoblastic leukemia.Cytotherapy(. 1:12–24. https://doi.org/10.1016/j.jcyt.2020.09.010
Yu L, Wei J (2022) Liu.Attacking the PI3K/Akt/mTOR signaling pathway for targeted therapeutic treatment in human cancer. Semin Cancer Biol 69–94. https://doi.org/10.1016/j.semcancer.2021.06.019
Evangelisti C, Chiarini F, Cappellini A et al (2020) Targeting Wnt/beta-catenin and PI3K/Akt/mTOR pathways in T-cell acute lymphoblastic leukemia. J Cell Physiol 6:5413–5428. https://doi.org/10.1002/jcp.29429
Spiegel JY, Patel S, Muffly L et al (2021) CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial.Nat Med. 8:1419–1431. https://doi.org/10.1038/s41591-021-01436-0
Shi J, Li Y, Jia R et al (2020) The fidelity of cancer cells in PDX models: characteristics, mechanism and clinical significance. Int J Cancer 8:2078–2088. https://doi.org/10.1002/ijc.32662
Jung J, Seol HS (2018) Chang.The Generation and Application of patient-derived xenograft model for Cancer Research.Cancer res treat. 1:1–10. https://doi.org/10.4143/crt.2017.307
Invrea F, Rovito R, Torchiaro E et al (2020) Patient-derived xenografts (PDXs) as model systems for human cancer.Curr Opin Biotechnol. 151–156. https://doi.org/10.1016/j.copbio.2020.01.003
Asnani M, Hayer KE, Naqvi AS et al (2020) Retention of CD19 intron 2 contributes to CART-19 resistance in leukemias with subclonal frameshift mutations in CD19.Leukemia(. 4:1202–1207. https://doi.org/10.1038/s41375-019-0580-z
Orlando EJ, Han X, Tribouley C et al (2018) Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat Med 10:1504–1506. https://doi.org/10.1038/s41591-018-0146-z
Höpken UE (2019) Rehm.Targeting the Tumor Microenvironment of Leukemia and Lymphoma.Trends Cancer. 6:351–364. https://doi.org/10.1016/j.trecan.2019.05.001
Zhang X, Zhu L, Zhang H et al CAR-T cell therapy in hematological malignancies: current Opportunities and Challenges.Front Immunol.(2022)927153.doi: https://doi.org/10.3389/fimmu.2022.927153
Funding
This work was supported in part by grants from the National Natural Science Foundation of China (grant nos. 81770202 and 81470304), Key Project from Science and Technology Department of Zhejiang Province (grant no:2019C03032), Pediatric Leukemia Diagnostic and Therapeutic Technology Research Center of Zhejiang Province (No. JBZX-201904). and the Zhejiang Medical and Health Science and Technology Plan Project (2022KY868).
Author information
Authors and Affiliations
Contributions
Diandian Ba, Hongzhe Li, Rongrong Liu ,and Ping Zhang performed the experiments. Diandian Ba and Hongzhe Li analyzed the data. Diandian Ba and Yongmin Tang wrote and revised the manuscript. All authors have contributed to the manuscript, reviewed the manuscript and approved the submitted version.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no conflicts of interests in this work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ba, D., Li, H., Liu, R. et al. Exploratory study on the efficacy of bortezomib combining mitoxantrone or CD22-CAR T therapy targeting CD19-negative relapse after CD19-CAR T cell therapy with a simpler cell-line-based model. Apoptosis 28, 1534–1545 (2023). https://doi.org/10.1007/s10495-023-01853-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-023-01853-1