Abstract
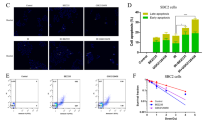
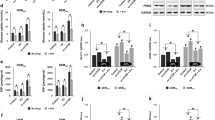
Cyclic nucleotide phosphodiesterase 5 (PDE5) has been recently identified to play a crucial role in the progression of many cancers. PDE5 promotes tumorigenesis by dysregulating various cellular processes such as proliferation, apoptosis, angiogenesis, and invasion and migration. Interestingly, multiple studies have reported the promising chemosensitizing potential of PDE5 inhibitor sildenafil in breast, colon, prostate, glioma, and lung cancers. However, to date, the chemosensitizing action of sildenafil is not evaluated in T cell lymphoma, a rare and challenging neoplastic disorder. Hence, the present investigation was undertaken to examine the chemosensitizing potential of sildenafil against T cell lymphoma along with elucidation of possible involvement of altered apoptosis and glucose metabolism. The experimental findings of this study showed that sildenafil enhances the cytotoxic ability of cisplatin by apoptosis induction through altering the levels of apoptosis regulatory molecules: Bcl-2, Bax, cytochrome c (Cyt c), cleaved caspase-3, and poly (ADP-ribose) polymerase (PARP). These molecular alterations were possibly driven by sildenafil through reactive oxygen species (ROS). Sildenafil deregulates glucose metabolism by markedly lowering the expression of glycolysis regulatory molecules, namely glucose transporter 1 (GLUT1), lactate dehydrogenase A (LDHA), hexokinase II (HKII), pyruvate kinase M2 (PKM2), and pyruvate dehydrogenase kinase 1 (PDK1) via suppressing hypoxia-inducible factor 1-alpha (HIF-1α) expression. Hence, sildenafil potentiates the tumor cell killing ability of cisplatin by augmenting ROS production through switching the glucose metabolism from glycolysis to oxidative phosphorylation (OXPHOS). Overall, our study demonstrates that sildenafil might be a promising adjunct therapeutic candidate in designing novel combinatorial chemotherapeutic regimens against T cell lymphoma.






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- ANOVA:
-
Analysis of variance
- BCIP/NBT:
-
5-Bromo-4-chloro-3′-indolyphosphate/nitro-blue tetrazolium
- DCFDA:
-
2′,7′-Dichlorofluorescin diacetate
- DL:
-
Dalton’s lymphoma
- EDTA:
-
Ethylenediaminetetraacetic acid
- FBS:
-
Fetal bovine serum
- FITC:
-
Fluorescein isothiocyanate
- GLUT1:
-
Glucose transporter 1
- HIF-1α:
-
Hypoxia-inducible factor 1-alpha
- HKII:
-
Hexokinase II
- LDHA:
-
Lactate dehydrogenase A
- MTT:
-
3-(4,5-Dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide
- PARP:
-
Poly (ADP-ribose) polymerase
- PBS:
-
Phosphate-buffered saline
- PDE5:
-
Phosphodiesterase 5
- PDK1:
-
Pyruvate dehydrogenase kinase 1
- PIPES:
-
Piperazine-N,N′-bis(2-ethanesulfonic acid)
- ROS:
-
Reactive oxygen species
- RPMI:
-
Roswell park memorial institute medium
- RT-PCR:
-
Reverse-transcription polymerase chain reaction
- SDS:
-
Sodium dodecyl sulphate
References
Niccolini F, Foltynie T, Reis Marques T et al (2015) Loss of phosphodiesterase 10A expression is associated with progression and severity in Parkinson’s disease. Brain 138:3003–3015. https://doi.org/10.1093/brain/awv219
Lorigo M, Oliveira N, Cairrao E (2021) PDE-mediated cyclic nucleotide compartmentation in vascular smooth muscle cells: from basic to a clinical perspective. J Cardiovasc Dev Dis. https://doi.org/10.3390/jcdd9010004
Yang HM, Jin S, Jang H et al (2019) Sildenafil reduces neointimal hyperplasia after angioplasty and inhibits platelet aggregation via activation of cGMP-dependent protein kinase. Sci Rep 9:7769. https://doi.org/10.1038/s41598-019-44190-7
Kurelic R, Krieg PF, Sonner JK et al (2021) Upregulation of phosphodiesterase 2A augments T cell activation by changing cGMP/cAMP cross-talk. Front Pharmacol 12:748798. https://doi.org/10.3389/fphar.2021.748798
Azevedo MF, Faucz FR, Bimpaki E et al (2014) Clinical and molecular genetics of the phosphodiesterases (PDEs). Endocr Rev 35:195–233. https://doi.org/10.1210/er.2013-1053
Barone I, Giordano C, Bonofiglio D, Ando S, Catalano S (2017) Phosphodiesterase type 5 and cancers: progress and challenges. Oncotarget 8:99179–99202. https://doi.org/10.18632/oncotarget.21837
Lin CS, Lin G, Xin ZC, Lue TF (2006) Expression, distribution and regulation of phosphodiesterase 5. Curr Pharm Des 12:3439–3457. https://doi.org/10.2174/138161206778343064
Catalano S, Campana A, Giordano C et al (2016) Expression and function of phosphodiesterase type 5 in human breast cancer cell lines and tissues: implications for targeted therapy. Clinical Cancer Res 22:2271–2282. https://doi.org/10.1158/1078-0432.CCR-15-1900
Li N, Chen X, Zhu B et al (2015) Suppression of beta-catenin/TCF transcriptional activity and colon tumor cell growth by dual inhibition of PDE5 and 10. Oncotarget 6:27403–27415. https://doi.org/10.18632/oncotarget.4741
Zhang N, Fang Z, Li Q et al (2017) PDE5 overexpression in well-differentiated thyroid carcinomas is associated with lymph node metastasis. Int J Endocrinol 2017:6243932. https://doi.org/10.1155/2017/6243932
Liu N, Mei L, Fan X et al (2016) Phosphodiesterase 5/protein kinase G signal governs stemness of prostate cancer stem cells through Hippo pathway. Cancer Lett 378:38–50. https://doi.org/10.1016/j.canlet.2016.05.010
Black KL, Yin D, Ong JM et al (2008) PDE5 inhibitors enhance tumor permeability and efficacy of chemotherapy in a rat brain tumor model. Brain Res 1230:290–302. https://doi.org/10.1016/j.brainres.2008.06.122
Murata K, Sudo T, Kameyama M et al (2000) Cyclic AMP specific phosphodiesterase activity and colon cancer cell motility. Clin Exp Metastasis 18:599–604. https://doi.org/10.1023/a:1011926116777
Ala M, Mohammad Jafari R, Dehpour AR (2021) Sildenafil beyond erectile dysfunction and pulmonary arterial hypertension: thinking about new indications. Fundam Clin Pharmacol 35:235–259. https://doi.org/10.1111/fcp.12633
Qian CN, Takahashi M, Kahnoski R, Teh BT (2003) Effect of sildenafil citrate on an orthotopic prostate cancer growth and metastasis model. J Urol 170:994–997. https://doi.org/10.1097/01.ju.0000080321.99119.df
Sarfati M, Mateo V, Baudet S et al (2003) Sildenafil and vardenafil, types 5 and 6 phosphodiesterase inhibitors, induce caspase-dependent apoptosis of B-chronic lymphocytic leukemia cells. Blood 101:265–269. https://doi.org/10.1182/blood-2002-01-0075
Booth L, Roberts JL, Cruickshanks N et al (2014) Phosphodiesterase 5 inhibitors enhance chemotherapy killing in gastrointestinal/genitourinary cancer cells. Mol Pharmacol 85:408–419. https://doi.org/10.1124/mol.113.090043
Das A, Durrant D, Mitchell C, Dent P, Batra SK, Kukreja RC (2016) Sildenafil (Viagra) sensitizes prostate cancer cells to doxorubicin-mediated apoptosis through CD95. Oncotarget 7:4399–4413. https://doi.org/10.18632/oncotarget.6749
Greish K, Fateel M, Abdelghany S et al (2018) Sildenafil citrate improves the delivery and anticancer activity of doxorubicin formulations in a mouse model of breast cancer. J Drug Target 26:610–615. https://doi.org/10.1080/1061186X.2017.1405427
El-Naa MM, Othman M, Younes S (2016) Sildenafil potentiates the antitumor activity of cisplatin by induction of apoptosis and inhibition of proliferation and angiogenesis. Drug Des Devel Ther 10:3661–3672. https://doi.org/10.2147/DDDT.S107490
Das A, Durrant D, Mitchell C et al (2010) Sildenafil increases chemotherapeutic efficacy of doxorubicin in prostate cancer and ameliorates cardiac dysfunction. Proc Natl Acad Sci USA 107:18202–18207. https://doi.org/10.1073/pnas.1006965107
Ghanbari Movahed Z, Rastegari-Pouyani M, Mohammadi MH, Mansouri K (2019) Cancer cells change their glucose metabolism to overcome increased ROS: one step from cancer cell to cancer stem cell? Biomed Pharmacother 112:108690. https://doi.org/10.1016/j.biopha.2019.108690
Redza-Dutordoir M, Averill-Bates DA (2016) Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta 1863:2977–2992. https://doi.org/10.1016/j.bbamcr.2016.09.012
Liberti MV, Locasale JW (2016) The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci 41:211–218. https://doi.org/10.1016/j.tibs.2015.12.001
Warburg O (1956) On the origin of cancer cells. Science 123:309–314. https://doi.org/10.1126/science.123.3191.309
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324:1029–1033. https://doi.org/10.1126/science.1160809
Marcucci F, Rumio C (2021) Glycolysis-induced drug resistance in tumors-a response to danger signals? Neoplasia 23:234–245. https://doi.org/10.1016/j.neo.2020.12.009
Tchounwou PB, Dasari S, Noubissi FK, Ray P, Kumar S (2021) Advances in our understanding of the molecular mechanisms of action of cisplatin in cancer therapy. J Exp Pharmacol 13:303–328. https://doi.org/10.2147/JEP.S267383
Karasawa T, Steyger PS (2015) An integrated view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicol Lett 237:219–227. https://doi.org/10.1016/j.toxlet.2015.06.012
Tang Q, Wang X, Jin H et al (2021) Cisplatin-induced ototoxicity: Updates on molecular mechanisms and otoprotective strategies. Eur J Pharm Biopharm 163:60–71. https://doi.org/10.1016/j.ejpb.2021.03.008
Jaiswara PK, Gupta VK, Sonker P et al (2021) Nimbolide induces cell death in T lymphoma cells: Implication of altered apoptosis and glucose metabolism. Environ Toxicol 36:628–641. https://doi.org/10.1002/tox.23067
Gupta VK, Jaiswara PK, Sonker P, Rawat SG, Tiwari RK, Kumar A (2020) Lysophosphatidic acid promotes survival of T lymphoma cells by altering apoptosis and glucose metabolism. Apoptosis 25:135–150. https://doi.org/10.1007/s10495-019-01585-1
Marbach EP, Weil MH (1967) Rapid enzymatic measurement of blood lactate and pyruvate. Use and significance of metaphosphoric acid as a common precipitant. Clin Chem 13:314–325
Mei XL, Yang Y, Zhang YJ et al (2015) Sildenafil inhibits the growth of human colorectal cancer in vitro and in vivo. Am J Cancer Res 5:3311–3324
Sponziello M, Verrienti A, Rosignolo F et al (2015) PDE5 expression in human thyroid tumors and effects of PDE5 inhibitors on growth and migration of cancer cells. Endocrine 50:434–441. https://doi.org/10.1007/s12020-015-0586-x
Klutzny S, Anurin A, Nicke B et al (2018) PDE5 inhibition eliminates cancer stem cells via induction of PKA signaling. Cell Death Dis 9:192. https://doi.org/10.1038/s41419-017-0202-5
Domvri K, Zarogoulidis K, Zogas N et al (2017) Potential synergistic effect of phosphodiesterase inhibitors with chemotherapy in lung cancer. J Cancer 8:3648–3656. https://doi.org/10.7150/jca.21783
Li Q, Shu Y (2014) Pharmacological modulation of cytotoxicity and cellular uptake of anti-cancer drugs by PDE5 inhibitors in lung cancer cells. Pharm Res 31:86–96. https://doi.org/10.1007/s11095-013-1134-0
Dasari S, Tchounwou PB (2014) Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 740:364–378. https://doi.org/10.1016/j.ejphar.2014.07.025
Kleih M, Bopple K, Dong M et al (2019) Direct impact of cisplatin on mitochondria induces ROS production that dictates cell fate of ovarian cancer cells. Cell Death Dis 10:851. https://doi.org/10.1038/s41419-019-2081-4
Tavallai M, Hamed HA, Roberts JL et al (2015) Nexavar/Stivarga and viagra interact to kill tumor cells. J Cell Physiol 230:2281–2298. https://doi.org/10.1002/jcp.24961
Beloueche-Babari M, Wantuch S, Casals Galobart T et al (2017) MCT1 Inhibitor AZD3965 increases mitochondrial metabolism, facilitating combination therapy and noninvasive magnetic resonance spectroscopy. Can Res 77:5913–5924. https://doi.org/10.1158/0008-5472.CAN-16-2686
Kittipongdaja W, Wu X, Garner J et al (2015) Rapamycin suppresses tumor growth and alters the metabolic phenotype in T-cell lymphoma. J Invest Dermatol 135:2301–2308. https://doi.org/10.1038/jid.2015.153
Kumar A, Kant S, Singh SM (2012) Novel molecular mechanisms of antitumor action of dichloroacetate against T cell lymphoma: Implication of altered glucose metabolism, pH homeostasis and cell survival regulation. Chem Biol Interact 199:29–37. https://doi.org/10.1016/j.cbi.2012.06.005
Kumar A, Kant S, Singh SM (2013) Antitumor and chemosensitizing action of dichloroacetate implicates modulation of tumor microenvironment: a role of reorganized glucose metabolism, cell survival regulation and macrophage differentiation. Toxicol Appl Pharmacol 273:196–208. https://doi.org/10.1016/j.taap.2013.09.005
Di X, Gennings C, Bear HD et al (2010) Influence of the phosphodiesterase-5 inhibitor, sildenafil, on sensitivity to chemotherapy in breast tumor cells. Breast Cancer Res Treat 124:349–360. https://doi.org/10.1007/s10549-010-0765-7
Hassanvand F, Mohammadi T, Ayoubzadeh N et al (2020) Sildenafil enhances cisplatin-induced apoptosis in human breast adenocarcinoma cells. J Cancer Res Ther 16:1412–1418. https://doi.org/10.4103/jcrt.JCRT_675_19
Gao JL, Chen YG (2015) Natural compounds regulate glycolysis in hypoxic tumor microenvironment. Biomed Res Int 2015:354143. https://doi.org/10.1155/2015/354143
Ikeda H, Kakeya H (2021) Targeting hypoxia-inducible factor 1 (HIF-1) signaling with natural products toward cancer chemotherapy. J Antibiot (Tokyo) 74:687–695. https://doi.org/10.1038/s41429-021-00451-0
Acknowledgements
The authors thankfully acknowledge fellowship support to Shiv Govind Rawat (Award No. 09/013(0772/2018-EMR-I)), Pradip Kumar Jaiswara (Award No. 1002/(SC)(CSIR-UGC NET DEC. 2016), and Vishal Kumar Gupta (Award No. 1044/(CSIR-UGC NET JUNE 2019) from CSIR, New Delhi. The fellowship supports to Rajan Kumar Tiwari (Award No. R/Dev/IX-Sch.(SRF-JRF-CAS-Zoology)/75159) from University Grants Commission-Career Advancement Scheme (UGC-CAS) is highly acknowledged. Funding from the Indian Council of Medical Research, New Delhi, India (Grant Number: IRIS ID-2021-13098) is highly acknowledged. The authors also acknowledge UGC-CAS and the Department of Science & Technology-Fund for Improvement of S&T Infrastructure (DST-FIST) program to the Department of Zoology, Banaras Hindu University, India. The authors thank Dr. Subhash Chandra Gupta (Department of Biochemistry, Banaras Hindu University, Varanasi) for providing the PARP antibody.
Funding
This work was supported by the Indian Council of Medical Research, New Delhi, India (Grant Number: IRIS ID-2021–13098).
Author information
Authors and Affiliations
Contributions
The research work presented in this manuscript is part of the PhD thesis of SGR. The experiments of this investigation were designed by AK and SGR. SGR has performed the entire experiments of the study. The manuscript was written by AK, SGR, NKV, SK, CP, and VG. SGR, RKT, PKJ, VKG, and PS prepared reagents. The experimental data were analyzed by AK, SGR, RKT, PKJ, VKG, PS, NKV, SK, CP, and VG. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rawat, S.G., Tiwari, R.K., Jaiswara, P.K. et al. Phosphodiesterase 5 inhibitor sildenafil potentiates the antitumor activity of cisplatin by ROS-mediated apoptosis: a role of deregulated glucose metabolism. Apoptosis 27, 606–618 (2022). https://doi.org/10.1007/s10495-022-01741-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-022-01741-0