Abstract
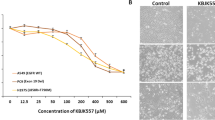
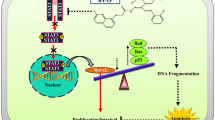
Reigning of the abnormal gene activation associated with survival signalling in lung cancer leads to the anomalous growth and therapeutic failure. Targeting specific cell survival signalling like JAK2/STAT3 nexus has become a major focus of investigation to establish a target specific treatment. The 2-bromobenzoyl-4-methylphenoxy-acetyl hydra acetyl Coumarin (BP-1C), is new anti-neoplastic agent with apoptosis inducing capacity. The current study was aimed to develop antitumor phramacophore, BP-1C as JAK2 specific inhibitor against lung neoplastic progression. The study validates and identifies the molecular targets of BP-1C induced cell death. Cell based screening against multiple cancer cell lines identified, lung adenocarcinoma as its specific target through promotion of apoptosis. The BP-1C is able to induce, specific hall marks of apoptosis and there by conferring anti-neoplastic activity. Validation of its molecular mechanism, identified, BP-1C specifically targets JAK2Tyr1007/1008 phosphorylation, and inhibits its downstream STAT3Tyr705 signalling pathway to induce cell death. As a consequence, modulation in Akt/Src survival signal and altered expression of interwoven apoptotic genes were evident. The results were reproducible in an in-vivo LLC tumor model and in-ovo xenograft studies. The computational approaches viz, drug finger printing confers, BP-1C as novel class JAK2 inhibitor and molecular simulations studies assures its efficiency in binding with JAK2. Overall, BP-1C is a novel JAK2 inhibitor with experimental evidence and could be effectively developed into a promising drug for lung cancer treatment.
Graphical abstract














Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information Files).
References
Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA (2008) Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 83(5):584–594. https://doi.org/10.4065/83.5.584
Solanki HS, Welsh EA, Fang B, Izumi V, Darville L, Stone B et al (2021) Cell type-specific adaptive signaling responses to KRASG12C inhibition. Clinical Cancer Res 27(9):2533–2548. https://doi.org/10.1158/1078-0432.CCR-20-3872
Sebastian M, Eberhardt W, Hoffknecht P, Metzenmacher M, Wehler T, Kokowski K, CRISP Registry Group et al (2021) KRAS G12C-mutated advanced non-small cell lung cancer: a real-world cohort from the German prospective, observational, nation-wide CRISP Registry (AIO-TRK-0315). Lung Cancer (Amsterdam, Netherlands) 154:51–61. https://doi.org/10.1016/j.lungcan.2021.02.005
Testa U, Castelli G, Pelosi E (2018) Lung cancers: molecular characterization, clonal heterogeneity and evolution, and cancer stem cells. Cancers 10(8):248. https://doi.org/10.3390/cancers10080248
Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB (2019) Inflammation and cancer. Ann Afr Med 18(3):121–126. https://doi.org/10.4103/aam.aam_56_18
Valavanidis A, Vlachogianni T, Fiotakis K, Loridas S (2013) Pulmonary oxidative stress, inflammation and cancer: respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int J Environ Res Public Health 10(9):3886–3907. https://doi.org/10.3390/ijerph10093886
Muthumalage T, Lamb T, Friedman MR, Rahman I (2019) E-cigarette flavored pods induce inflammation, epithelial barrier dysfunction, and DNA damage in lung epithelial cells and monocytes. Sci Rep 9(1):19035. https://doi.org/10.1038/s41598-019-51643-6
Owen KL, Brockwell NK, Parker BS (2019) JAK-STAT signaling: a double-edged sword of immune regulation and cancer progression. Cancers (Basel) 11(12):2002. https://doi.org/10.3390/cancers11122002
Banerjee S, Biehl A, Gadina M, Hasni S, Schwartz DM (2017) JAK-STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs 77(5):521–546. https://doi.org/10.1007/s40265-017-0701-9
Patel MR, Dash A, Jacobson BA, Ji Y, Baumann D, Ismail K et al (2019) JAK/STAT inhibition with ruxolitinib enhances oncolytic virotherapy in non-small cell lung cancer models. Cancer Gene Ther 26(11–12):411–418. https://doi.org/10.1038/s41417-018-0074-6
Sun Y, Zhou P, Chen S, Hu C, Bai Q, Wu H et al (2017) The JAK/STAT3 signaling pathway mediates inhibition of host cell apoptosis by Chlamydia psittaci infection. Pathog Dis. https://doi.org/10.1093/femspd/ftx088
Guru SA, Sumi MP, Mir R, Waza AA, Bhat MA, Zuberi M et al (2020) Ectopic PD-L1 expression in JAK2 (V617F) myeloproliferative neoplasm patients is mediated via increased activation of STAT3 and STAT5. Hum Cell 33(4):1099–1111. https://doi.org/10.1007/s13577-020-00370-6
Morgan EL, Andrew M (2019) JAK2 inhibition impairs proliferation and sensitises cervical cancer cells to cisplatin-induced cell death. Cancers (Basel) 11(12):1934. https://doi.org/10.3390/cancers11121934
Lin TE, HuangFu WC, Chao MW, Sung TY, Chang CD, Chen YY et al (2018) A novel selective JAK2 inhibitor identified using pharmacological interactions. Front Pharmacol 9:1379. https://doi.org/10.3389/fphar.2018.01379
Wu Y, Xu J, Liu Y, Zeng Y, Wu G (2020) A review on anti-tumor mechanisms of coumarins. Front Oncol 10:592853. https://doi.org/10.3389/fonc.2020.592853
Musa MA, Cooperwood JS, Khan MO (2008) A review of coumarin derivatives in pharmacotherapy of breast cancer. Curr Med Chem 15(26):2664–2679. https://doi.org/10.2174/092986708786242877
Prashanth T, VijayAvin BR, Thirusangu P, Ranganatha VL, Prabhakar BT, Sharath Chandra JNN, Khanum SA (2019) Synthesis of coumarin analogs appended with quinoline and thiazole moiety and their apoptogenic role against murine ascitic carcinoma. Biomed Pharmacother 112:108707. https://doi.org/10.1016/j.biopha.2019.108707
Dong MX, Ye T, Bi YY, Wang Q, Kuerban KDLD, Li JY et al (2019) A novel hybrid of 3-benzyl coumarin seco-B-ring derivative and phenylsulfonylfuroxan induces apoptosis and autophagy in non-small-cell lung cancer. Phytomedicine 52:79–88. https://doi.org/10.1016/j.phymed.2018.09.216
Prabhakar BT, Khanum SA, Jayashree K, Salimath BP, Shashikanth S (2006) Anti-tumor and proapoptotic effect of novel synthetic benzophenone analogues in Ehrlich ascites tumor cells. Bioorg Med Chem 14(2):435–446. https://doi.org/10.1016/j.bmc.2005.08.039
Thirusangu P, Vigneshwaran V, Prashanth T, VijayAvin BR, Malojirao VH, Rakesh H et al (2017) BP-1T, an antiangiogenic benzophenone-thiazole pharmacophore, counteracts HIF-1 signalling through p53/MDM2-mediated HIF-1α proteasomal degradation. Angiogenesis 20(1):55–71. https://doi.org/10.1007/s10456-016-9528-3
Zabiulla SHG, Bushra BA, Prabhakar BT, Khanum SA (2016) Design and synthesis of diamide-coupled benzophenones as potential anticancer agents. Eur J Med Chem 15:342–351. https://doi.org/10.1016/j.ejmech.2016.03.040
Preeyaporn PP, Kesarin B, Chuanpit N, Pithi C (2017) Benzophenone-3 increases metastasis potential in lung cancer cells via epithelial to mesenchymal transition. Cell Biol Toxicol 33(3):251–261. https://doi.org/10.1007/s10565-016-9368-3
VijayAvin BR, Thirusangu P, Lakshmi RV, Firdouse A, Prabhakar BT, Khanum SA (2014) Synthesis and tumor inhibitory activity of novel coumarin analogs targeting angiogenesis and apoptosis. Eur J Med Chem 75:211–221. https://doi.org/10.1016/j.ejmech.2014.01.050
Malojirao VH, Vigneshwaran V, Thirusangu P, Mahmood R, Prabhakar BT (2018) The tumor antagonistic steroidal alkaloid Solanidine prompts the intrinsic suicidal signal mediated DFF-40 nuclear import and nucleosomal disruption. Life Sci 199:139–150. https://doi.org/10.1016/j.lfs.2018.03.015
Malojirao VH, Girimanchanaika SS, Shanmugam MK, Ankith S, Dukanya MPK et al (2020) Novel 1,3,4-oxadiazole targets STAT3 signaling to induce antitumor effect in lung cancer. Biomedicines 8(9):368. https://doi.org/10.3390/biomedicines8090368
Matatall KA, Kadmon CS, King KY (2018) Detecting hematopoietic stem cell proliferation using BrdU incorporation. Methods Mol Biol 1686:91–103. https://doi.org/10.1007/978-1-4939-7371-2_7
Møller P (2018) The comet assay: ready for 30 more years. Mutagenesis 33(1):1–7. https://doi.org/10.1093/mutage/gex046
Chazotte B (2011) Labeling mitochondria with MitoTracker dyes. Cold Spring Harb Protoc 8:990–992. https://doi.org/10.1101/pdb.prot5648
Sys GM, Lapeire L, Stevens N, Favoreel H, Forsyth R, Bracke M et al (2013) The in ovo CAM-assay as a xenograft model for sarcoma. J Vis Exp 77:e50522. https://doi.org/10.3791/50522
Fares HA, Ankith S, Vigneshwaran V, Giridhara B, Vivek HK, Prabhakar BT, Khanum SA (2021) Targeting HIF-1α by newly synthesized indolephenoxyacetamide (IPA) analogs to induce anti-angiogenesis-mediated solid tumor suppression. Pharmacol Rep. https://doi.org/10.1007/s43440-021-00266-8
Thirusangu P, Vigneshwaran V, Ranganatha VL, VijayAvin BR, Khanum SA, Mahmood R, Jayashree K, Prabhakar BT (2017) A tumoural angiogenic gateway blocker, Benzophenone-1B represses the HIF-1α nuclear translocation and its target gene activation against neoplastic progression. Biochem Pharmacol 125:26–40. https://doi.org/10.1016/j.bcp.2016.11.009
RDKit: Open-source cheminformatics. Greg Landrum ACS, San Diego
Rogers D, Hahn M (2010) Extended-connectivity fingerprints. J Chem Inf Model 50(5):742–754
Mendez D, Gaulton A, Patrícia BA, Chambers J et al (2019) ChEMBL: towards direct deposition of bioassay data. Nucleic Acids Res. https://doi.org/10.1093/nar/gky1075
Waskom M, Botvinnik O, O’Kane D, Hobson P, Lukauskas S, Gemperline DC, Augspurger T, Halchenko Y, Cole JB, Warmenhoven J, de Ruiter J, Pye C, Hoyer S, Vanderplas J, Villalba S, Kunter G, Quintero E, Bachant P, Martin M, Meyer K, Miles A, Ram Y, Yarkoni T, Williams ML, Evans C, Qalieh A (2017) mwaskom/seaborn: V0.8.1 (2017). Zenodo. https://doi.org/10.5281/zenodo.883859
Hunter JD (2007) Matplotlib: a 2D graphics environment. Comput Sci Eng 9:99–104. https://doi.org/10.1109/MCSE.2007.55
O’Boyle NM, Banck M, James CA et al (2011) Open babel: an open chemical toolbox. J Cheminform 3:33. https://doi.org/10.1186/1758-2946-3-33
Morris GM, Ruth H, Lindstrom W et al (2009) Software news and updates AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30:2785–2791. https://doi.org/10.1002/jcc.21256
Brasca MG, Gnocchi P, Nesi M et al (2015) Novel pyrrole carboxamide inhibitors of JAK2 as potential treatment of myeloproliferative disorders. Bioorg Med Chem 23(10):2387–2407. https://doi.org/10.1016/j.bmc.2015.03.059
Case DA, Betz RM, Cerutti DS et al (2016) AMBER 2016 reference manual. University of California, San Francisco
Maier JA, Martinez C, Kasavajhala K, Wickstrom L, Hauser KE, Simmerling C (2015) ff14SB: improving the accuracy of protein side chain and backbone parameters from ff99SB. J Chem Theory Comput 11(8):3696–3713
Genheden S, Ryde U (2015) The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin Drug Discov 10(5):449–461
Gohlke H, Kiel C, Case DA (2003) Insights into protein-protein binding by binding free energy calculation and free energy decomposition for the Ras-Raf and Ras-RalGDS complexes. J Mol Biol 330(4):891–913
Dong J, Wang NN, Yao ZJ et al (2018) ADMETlab: a platform for systematic ADMET evaluation based on a comprehensively collected ADMET database. J Cheminform 10:29. https://doi.org/10.1186/s13321-018-0283-x
Lipinski Christopher A (2004) Lead- and Drug-like Compounds: the rule-of-five evolution. Drug Discov Today Technol 1(4):337–341. https://doi.org/10.1016/j.ddtec.2004.11.007
Liu JF, Deng WW, Chen L, Li YC, Wu L, Ma SR et al (2018) Inhibition of JAK2/STAT3 reduces tumor-induced angiogenesis and myeloidderived suppressor cells in head and neck cancer. Mol Carcinog 57(3):429–439. https://doi.org/10.1002/mc.22767
Hobbs GS, Rozelle S, Mullally A (2017) The development and use of janus kinase 2 inhibitors for the treatment of myeloproliferative neoplasms. Hematol Oncol Clin N Am 31:613–626. https://doi.org/10.1016/j.hoc.2017.04.002
Xu Y, Jin J, Xu J, Shao YW, Fan Y (2017) JAK2 variations and functions in lung adenocarcinoma. Tumour Biol 39(6):1010428317711140. https://doi.org/10.1177/1010428317711140
Artaş G, Ozercan HI (2014) The expression of STAT3, BCL-XL and MMP-2 proteins in colon adenocarcinomas and their relationship with prognostic factors. Turk Patol Derg 30(3):178–183. https://doi.org/10.5146/tjpath.2014.01269
Jiang W, Xu Z, Yu L, Che J, Zhang J, Yang J (2019) MicroRNA-144-3p suppressed TGF-β1-induced lung cancer cell invasion and adhesion by regulating the Src-Akt-Erk pathway. Cell Biol Int. https://doi.org/10.1002/cbin.11158
Korshunov DA, Klimov IA, Ivanov VV, Kondakova IV (2018) Glycolysis inhibitors monoiodoacetate and 2-deoxyglucose as antitumor agents: experimental study on Lewis lung carcinoma model. Bull Exp Biol Med 165(5):695–697. https://doi.org/10.1007/s10517-018-4244-1
Acknowledgements
Ankith Sherapura thankful to the Lady Tata Memorial Trust for JRS (2020–2021) and B.T. Prabhakar gratefully acknowledge the grant support by VGST (VGST/P-2/CISEE/GRD-231/2013–14) and SERB [EMR/2017/00088/ dated 3/01/2019]. Shaukath Ara Khanum thankfully acknowledges the financial support provided by VGST, Bangalore, under CISEE Program [Project Sanction Order No. VGST/CISEE/282/2012-13].
Author information
Authors and Affiliations
Contributions
AS performed all the experiments, VHM, TP, VV assisted in experimental setup, and S.B.S, S.K, JN supported through in-silico approach, LR for synthesis of drug, SB, for scientific discussion and SAK and PBT designed the experiments, wrote the manuscript and monitored the entire investigation.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.




Rights and permissions
About this article
Cite this article
Sherapura, A., Malojirao, V.H., Thirusangu, P. et al. Anti-neoplastic pharmacophore benzophenone-1 coumarin (BP-1C) targets JAK2 to induce apoptosis in lung cancer. Apoptosis 27, 49–69 (2022). https://doi.org/10.1007/s10495-021-01699-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-021-01699-5