Abstract
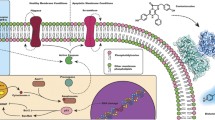
ANNEXIN V belongs to a family of phospholipid binding proteins which is able to bind to negatively charged phospholipids such as phosphatidylserine (PS) in the presence of a high affinity Ca2+ ion. When apoptosis occurs, even at early stage, PS will be exposed to the outside of the cell surface from the cytoplasm side of membrane leaflets., Therefore ANNEXIN V has been suggested as a bio-marker for imaging early apoptotic events of various cell death including those in disease conditions. However, most ANNEXIN V-based apoptotic detecting techniques were in vitro approaches. Here, we presented a new BRET (Bioluminescence Resonance Energy Transfer) based genetic coded biosensor by fusing ANNEXIN V and a BRET version of NanoLuc (teLuc) for both in vitro and in vivo apoptosis detection. The BRET feature of this new sensor makes it convenient to be applied to both conventional fluorescent-based in vitro apoptosis detection and bioluminescence-based animal live imaging for in vivo study. Because of its robust bioluminescence signal, it is possible to perform the evaluation of the disease-induced apoptotic damage and recovery process directly at deep tissue level in live animal.







Similar content being viewed by others
References
Blankenberg FG (2008) Monitoring of treatment-induced apoptosis in oncology with PET and SPECT. Curr Pharm Des 14:2974–2982
Wolters SL, Corsten MF, Reutelingsperger CP, Narula J, Hofstra L (2007) Cardiovascular molecular imaging of apoptosis. Eur J Nucl Med Mol Imaging 34(Suppl 1):S86-98
Laufer EM, Winkens HM, Corsten MF, Reutelingsperger CP, Narula J, Hofstra L (2009) PET and SPECT imaging of apoptosis in vulnerable atherosclerotic plaques with radiolabeled Annexin A5. Q J Nucl Med Mol Imaging 53:26–34
Bahmani P, Schellenberger E, Klohs J et al (2011) Visualization of cell death in mice with focal cerebral ischemia using fluorescent annexin A5, propidium iodide, and TUNEL staining. J Cereb Blood Flow Metab 31:1311–1320
Post AM, Katsikis PD, Tait JF, Geaghan SM, Strauss HW, Blankenberg FG (2002) Imaging cell death with radiolabeled annexin V in an experimental model of rheumatoid arthritis. J Nucl Med 43:1359–1365
Head T, Dau P, Duffort S et al (2017) An enhanced bioluminescence-based Annexin V probe for apoptosis detection in vitro and in vivo. Cell Death Dis 8:e2826
Raynal P, Pollard HB (1994) Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim Biophys Acta 1197:63–93
Swairjo MA, Concha NO, Kaetzel MA, Dedman JR, Seaton BA (1995) Ca(2+)-bridging mechanism and phospholipid head group recognition in the membrane-binding protein annexin V. Nat Struct Biol 2:968–974
Logue SE, Elgendy M, Martin SJ (2009) Expression, purification and use of recombinant annexin V for the detection of apoptotic cells. Nat Protoc 4:1383–1395
Brumatti G, Sheridan C, Martin SJ (2008) Expression and purification of recombinant annexin V for the detection of membrane alterations on apoptotic cells. Methods 44:235–240
Blankenberg FG, Tait JF, Blankenberg TA, Post AM, Strauss HW (2001) Imaging macrophages and the apoptosis of granulocytes in a rodent model of subacute and chronic abscesses with radiolabeled monocyte chemotactic peptide-1 and annexin V. Eur J Nucl Med 28:1384–1393
Haas RL, de Jong D, Valdes Olmos RA et al (2004) In vivo imaging of radiation-induced apoptosis in follicular lymphoma patients. Int J Radiat Oncol Biol Phys 59:782–787
Belhocine T, Steinmetz N, Hustinx R et al (2002) Increased uptake of the apoptosis-imaging agent (99m)Tc recombinant human Annexin V in human tumors after one course of chemotherapy as a predictor of tumor response and patient prognosis. Clin Cancer Res 8:2766–2774
Narula J, Acio ER, Narula N et al (2001) Annexin-V imaging for noninvasive detection of cardiac allograft rejection. Nat Med 7:1347–1352
Ntziachristos V, Schellenberger EA, Ripoll J et al (2004) Visualization of antitumor treatment by means of fluorescence molecular tomography with an annexin V-Cy5.5 conjugate. Proc Natl Acad Sci U S A 101:12294–12299
Kain SR, Ma JT (1999) Early detection of apoptosis with annexin V-enhanced green fluorescent protein. Methods Enzymol 302:38–43
Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C (1995) A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods 184:39–51
Martin SJ, Reutelingsperger CP, McGahon AJ et al (1995) Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med 182:1545–1556
Hall MP, Unch J, Binkowski BF et al (2012) Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS Chem Biol 7:1848–1857
Yeh HW, Karmach O, Ji A, Carter D, Martins-Green MM, Ai HW (2017) Red-shifted luciferase-luciferin pairs for enhanced bioluminescence imaging. Nat Methods 14:971–974
Suzuki K, Kimura T, Shinoda H et al (2016) Five colour variants of bright luminescent protein for real-time multicolour bioimaging. Nat Commun 7:13718
Tait JF, Smith C, Blankenberg FG (2005) Structural requirements for in vivo detection of cell death with 99mTc-annexin V. J Nucl Med 46:807–815
Labat-gest V, Tomasi S (2013) Photothrombotic ischemia: a minimally invasive and reproducible photochemical cortical lesion model for mouse stroke studies. J Vis Exp. https://doi.org/10.3791/50370
Acknowledgements
We thank Dr. Xiaoxuan Xu for technique support when conducting live imaging. We thank Mr. Zhikang Zhao for helping animal care and maintenance. This work was supported by the National Key Basic Research Program (NKBRP) (BK20130057) from Ministry of Science and Technology of the People's Republic of China and the National Natural Science Foundation of China. (Grant # 3224003212).
Funding
National Key Basic Research Program (NKBRP) (BK20130057) from Ministry of Science and Technology of the People's Republic of China and the National Natural Science Foundation of China. (Grant # 3224003212).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
The study was approved by the Institutional Animal Use and Care Committee of Southeast University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10495_2021_1693_MOESM1_ESM.tif
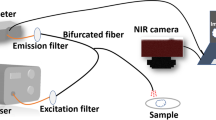
BRET property of AnVPb and AnV-Antares2 probes. Bioluminescence emission signal of lysis supernatants from 9 individual clones for each probe was collected after mixed with substrate DTZ (30 µM). Averaged bioluminescence curves of AnVPb (a) and AnV-Antares2 (b) demonstrated a much higher activity (>100 times) in AnVPb supernatant. Individual bioluminescence curves of AnVPb clones (c) presented an identical emission curve peaked at 516 nm. While the BRET curves of AnV-Antares2 clones varied a lot, indicating a less stability of AnV-Antares2 (tif 3249 kb)
Rights and permissions
About this article
Cite this article
Zhang, Y., Hu, J., Yu, M. et al. A novel BRET based genetic coded biosensor for apoptosis detection at deep tissue level in live animal. Apoptosis 26, 628–638 (2021). https://doi.org/10.1007/s10495-021-01693-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-021-01693-x